Abstract
Within this paper the influence of the process parameters time, temperature and pH on properties and composition of hydrochars from anaerobic digestate is investigated using statistical methods. Response surface method was applied to assess the optimal process conditions for nutrient recovery. Additionally, the chemical structure of several hydrochar samples was analysed via solid state NMR to make statements about the process parameters influence on the built up of the char. It was shown that optimal conditions for carbonization and for nutrient recovery require conditions that are divergent in temperature and pH. Carbonization is maximized at a temperature of 230 °C and pH of around 3. Phosphorus recovery shows its highest values at a pH of 8 and a temperature of 165 °C. Nitrogen recovery is maximized at a pH of 3 and a temperature of 165 °C. Maximized nitrogen recovery at a low pH is opposite to the trend expected. This result is explained by the pH and temperature dependent equilibrium of solved ammonium and free ammonia. Changes in the chemical structure of the hydrochar start at carbonization-temperature of 220 °C. The results show that cellulose could be completely degraded and anaerobic digestate could be fully carbonized at severe reaction conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past 200 years agricultural intensification has led to a substantial increase in crop production ensuring nourishment of a constantly growing world population. As one of the side effects of this modus operandi, the intensive use of nutrients led to environmental pollution and political dependency on fertilizers especially in the western countries. A main driver behind agricultural intensification is the use of chemical fertilizers and manures. However, the usage of especially nitrogen and phosphorus rich fertilizers is dispersed unequally seen from a global scheme of things. 32% of the global nitrogen surplus and 40% of the global phosphorus surplus are caused by the croplands of the developed countries, while they represent only 10% of the worlds croplands [1].
Due to rapid population growth and the increased food demand that is linked to it, fertilization in the form of excreta from humans and animals was not sufficient any more in the seventeenth and eighteenth century hence it got enriched with nutrients from other sources. In the nineteenth century mining of phosphate-rich rock began and up to today it ensures global food production. 90% of the worlds phosphorus demand is for food production and the demand is increasing. At the same time phosphorus is non-renewable and no phosphorus rock reserves exist in Europe indicating a high dependency of countries such as China, Marocco and the USA [2].
The human inputs of nitrogen are mainly due to fertilization, but also deposition from fossil fuel burning has a severe impact as well as the human caused biological fixation of atmospheric N2 by leguminous crop cultivation. Nitrogen for industrial fertilizers is produced via the energy-intensive Haber-Bosch-process. While enough nitrogen is available its use is inefficient. Only 22% of the total human inputs end up in soil and biomass, the rest is deposited in water bodies. The use of nitrogen fertilizer has increased tenfold since the 1950s and is still increasing [3]. Most the planets nitrogen exists as inactive gas. In its reactive form, it is a plant nutrient but at the same time it can act as a pollutant, when present in excessive amount. It can lead to destabilisation of ecosystems, as rapidly growing, nitrophilous plant species outcompete other species. Reactive nitrogen compounds also lead to development of ground-level ozone that is a considerable stress factor for plants. Additionally, excess nitrogen can end up in groundwater sources where drinking water originates from. In water bodies reactive nitrogen can also lead to eutrophication, when present in excess amounts [4].
It is evident that nutrient recycling is necessary to ensure sustainable resource utilization, to reduce environmental pollution and the western world’s dependency of politically unstable regions providing resources. It is necessary to reduce nutrients where they are unwanted (waste streams) and bring them to places where their existence is beneficial (fertilizer on field). Especially for waste streams with a high water content that is not always easy. Because of the high transport costs, the solid phase has to be separated from the aqueous phase. Solid–liquid separation still is energy-, hence also cost-intensive. Especially for biogas plants that leads to environmental implications. The anaerobic digestate from biogas production is still high in nutrients and usually complies with the European organic matter minimal requirement for an organic soil amendment. Due to its high water content and its high transportation costs it is usually deployed to the nearby fields. That frequently leads to over-fertilization, especially in the case of nitrogen. That causes nitrogen-loss, primarily via denitrification followed by emission of reactive nitrogen into the atmosphere and also via loss into water bodies. A common problem in Germany is, that in areas where there are many biogas plants, also massive amounts of manure from the Netherlands are used as soil fertilizer. The soil already is out of its nitrogen balance and use of biogas digestate intensifies the problem [5].
Applying hydrothermal carbonization on anaerobic digestate from biogas production has the potential to solve several problems at once. Bacterial pathogens as well as spores are killed during the process, hence the sanitation step happens within the process. The resulting hydrochar is more hydrophobic than the anaerobic digestate, hence dewatering is less energy intensive [6]. Partitioning of nutrients can be controlled during the process, it can either be primarily in the liquid phase, the process water, or primarily in the solid phase, the hydrochar, or split between the two compounds [7, 8]. The process water can then be applied on the nearby fields whereas the hydrochar can be transported to areas further away. This way also the nitrogen input into soils can be controlled to a certain degree. While certain problems can be solved via hydrothermal carbonization, the environmental impact of hydrochar application on soil can be disadvantageous. Phytotoxic effects of compounds that are formed during the process, like PAH and phenols, have been reported. Bargmann et al. reported an inhibition of germination of spring barley and cress when hydrochar or process water was added. This inhibition coulds be reduced by pre-treatment of the hydrochars (washing, storage) [9].
Hydrothermal Carbonization
Hydrothermal carbonization (HTC) is a thermal process converting different organic feedstocks into coal-like products in the presence of water under subcritical conditions. Recently HTC became a process more widely recognized and different purposes for its coal-like products have been discussed, including its use as fertilizer [10]. Other utilization routes could be as fuel for combustion plants or as activated charcoal. By applying temperatures between 180 and 250 °C on mixtures of biomass and water for several hours the hydrogen and oxygen contents of the feed are reduced, while its carbon content and energy density are increased [11]. Also gas is produced during the process, but its amount is negligible compared to other thermos-chemical conversion processes like pyrolysis. To characterize the degree of carbonization the H/C- and O/C-ratios of the feed material and the hydrochar are commonly displayed in van Krevelen diagrams that were established initially to characterize coal. The higher the difference between H/C- and O/C- ratios between feed and hydrochar, the more the feed material is carbonized. Between its boiling point at 100 °C and its critical point at 374 °C pressurized water is present under subcritical conditions. It then acts as a solvent, reactant and catalyst [12]. Different properties of water are temperature-dependent, like dielectric constant, density, viscosity, diffusion coefficient and ion product [13]. The dielectric constant permanently decreases with rising temperature due to the decreased effectiveness of hydrogen bonds. Water becomes less polar and acts more like a solvent. Organic molecules then show increased solubility, also because of the organic molecules higher enthalpy of solution. The ion product increases with rising temperature, hence the rate of acid or base influenced reactions increases [14]. During hydrothermal carbonization the biomass is hydrolysed into its monomers. Resulting sugars are solved in the process water and are converted into other compounds, like furfurals. The furfurals condense and form aromatic ring compounds [15]. This form of carbon is more stable and hydrochar can act as a carbon sink when applied to fields [11]. It is assumed that via the O/C-ratio in the hydrochar, statements about its stability as a carbon sink can be made [16].
Objective
The main purpose of this study is to investigate the effects of the process parameters time, temperature and pH on carbon and nutrient recovery as well as on the degree of carbonization of anaerobic digestate with high water content (95 wt%) from biogas production. Previous works designated temperature as the parameter with the highest impact on composition and built-up of hydrochar from anaerobic digestate [17, 18]. Within this work a clear description of the impact of the process parameters (time, temperature, pH) is made. It is clarified which process parameters influence the different properties of the hydrochar most and in how far the different process parameters influence each other. That gives valuable information for process control, as dependencies and interactions of process parameters and hydrochar characteristics are shown. It is expected, that especially for nutrient recovery a low temperature and a high pH are beneficial. As the purpose of HTC-char usage is either as solid fuel or as soil amendment the optimized process parameters are computed for those two scenarios. To further simplify the process and reduce extra energy input, separation of process water and hydrochar was conducted with a sieve with a mash size of 1 mm. The assumption was, that no elaborated filtration methods are necessary to achieve useful dry mass recoveries of hydrochars.
Hydrothermal carbonization of anaerobic digestate could be highly beneficial. Hydrothermal carbonization can increase dewaterability, pathogen destruction and to a certain extent provide nutrient recovery [19–22].
Within this work it is determined to what degree the process parameters influence the characteristics of the resulting hydrochar. Design of experiments (DOE) was used to establish a plan of experiments and to evaluate the data acquired from analysis of the different hydrochars samples. By that, the effects of the process parameters time, temperature and pH on the target parameters carbon recovery, dry mass recovery, difference of O/C-ratios between feed and hydrochar, nitrogen recovery and phosphorus recovery are assessed. The effect of the process parameters on the target parameters are displayed in main effects charts, showing the mean values of a target parameter at a certain process parameter. This way the general trend of the influence of a process parameter on a target parameter is shown. Additionally, the optimal process conditions are computed applying response surface design. As an experimental plan a central composite design was realized. Second degree polynomial models to describe the target parameters were established that can be optimized.
Citric acid was used to adjust the pH. The amount of citric acid needed to reduce the pH to 3.3 is high, equaling the amount mass dry mass in the anaerobic digestate. Therefore, the added citric acid is considered as part of the feed material. The carbon-, the oxygen-, the hydrogen content and the weight of the citric acid were considered as part of the feed material when carbon recovery and dry mass recovery were calculated.
Materials and Methods
Reactor for Hydrothermal Carbonization
A HTC-Reactor out of stainless steel is located at the Institute of Chemical and Energy Engineering at University of Natural Resources and Life Sciences, Vienna. It comprises a volume of 1.2 L. On the blind flange on top of the reactor temperature sensor (PT 100 from TC direct), pressure sensor (Unik 5000 from General Electrics) and safety valve (Götze Armaturen) are mounted. The reactor is heated with a heating element (3 kW, from RS components) that is coiled around and fixed on the reactor. The cooling system consists of a copper tube that is coiled around the reactor (underneath the heating). On the two ends of the copper tube two cooling hoses are mounted that are connected to the cooling system of the technical centre that delivers water with a temperature of 11 °C. The reactor is located in a metal cube and is isolated with rock wool. Pressure and temperature sensors are connected to a programmable logic controller (PLC). Via the PLC temperature and pressure are recorded during the experiments.
Anaerobic Digestate
The anaerobic digestate originates form a biogas production plant in Bruck/Leitha in Lower Austria. The feed material for biogas production are food wastes and agricultural wastes. The digestate has a dry mass of about 5 wt% and a pH of 8. The anaerobic digestate consists of a solid phase and solved compounds. One carbonization experiment was executed with an anaerobic digestate from agricultural waste only. It also has a dry mass of about 5 wt% and a pH of 8. It was carried out to gather information about the built-up of the hydrochar and is not included in the trial series that was evaluated statistically.
Carbonization Experiments
The anaerobic digestate was stored in containers of 1.5 L in the freezer at −18 °C and was thawed overnight. Before the experiment, the slurry was homogenized, by separating the solid part from the liquid part with a sieve (mash size 1 mm). Then the solid and the liquid part were weighed. One kilogram of anaerobic digestate was prepared by mixing solid and liquid part with the same ratio as the original material. If a pH reduction was desired, citric acid was added to the anaerobic digestate until the pH had a value of 3.3 or 5.5 respectively. Then the reactor was filled with the slurry, closed and was placed in the holding device. The cables of the sensors were connected to the programmable logic controller and the reactor was heated. The carbonization time is the time from the point when the reactor reached its desired temperature until the point when the cooling system is turned on. After cooling the reactor was emptied, the product was weighed and separated into hydrochar and process water with a sieve (1 mm). These products were weighed again. The hydrochar was air-dried for 7–11 days and then dried at 105 °C overnight. Then it was milled with a mortar, sieved to a size < 0.5 mm and stored in the freezer at −18 °C. The feed material that was not carbonized in the reactor was dried at 105 °C, milled, sieved (<0.5 mm) and stored at −18 °C. Hence each carbonization experiment has its own feed. The hydrochar from every experiment was compared to its feed.
Analytics
Elemental Analysis
Elemental analysis of C, H, N, S and O was performed using a Eurovector EA 3000 CHNS-O Elemental Analyser (built 2009). Phosphorus analysis was conducted via UV-vis-Spectrometry. The samples were treated with concentrated sulfuric acid and the obtained phosphates were quantified with molybdenum blue.
NMR Spectroscopy
All solid state nmr experiments were performed on a Bruker Avance III HD 400 spectrometer (resonance frequency of 1H of 400.13, 13C of 100.61, 15N of 40.58 MHz respectively), equipped with a 4 mm dual broadband CP-MAS probe. 13C spectra were acquired by using the TOSS (total sideband suppression) sequence at ambient temperature with a spinning rate of 5 kHz, a cross-polarization (CP) contact time of 2 ms, a recycle delay of 2 s, SPINAL-64 1H decoupling. 2k data points were sampled with an acquisition time of 43 ms resulting in a total sweep width of 240 ppm. Chemical shifts were referenced externally against the carbonyl signal of glycine with δ = 176.03 ppm. The acquired FIDs were apodized with an exponential function (lb = 11 Hz) prior to Fourier transformation.
CP/MAS 15N spectra were acquired with a spinning rate of 8 kHz, a cross-polarization (CP) contact time of 2 ms, a recycle delay of 2 s, SPINAL-64 1H decoupling and an acquisition time of 35 ms whereas the spectral width was set to 600 ppm. Chemical shifts were referenced externally against the 15N signal of glycine with δ = −346 ppm. The acquired FIDs were apodized with an exponential function (lb ≥ 11 Hz) prior to Fourier transformation.
Design of Experiment (DOE)
DOE was conducted with Statgraphics XVII (64 bit). Process parameters are time, temperature and pH. Target parameters are carbon recovery, dry mass recovery, nitrogen recovery, phosphorus recovery and difference in O/C-ratios between feed and char (see Table 1). The response surface method was applied, to obtain an optimal response. A second degree polynomial was chosen, that can be optimized [23]:
where t refers to carbonization time, T to temperature and A–J are the regression coefficients.
Concerning the type of design, a central composite design was chosen. It consists of a central point, corner points within the margins that are specified (see Table 1) and extreme points (see Fig. 1). There are three extreme points, two regarding temperature, one regarding time, all present at a pH of 5.5. All the experiments with their corresponding carbonization temperature, time and pH are displayed in Table 2. A suggested low extreme point regarding a pH of 2 could not be realized, because citric acid is a weak acid. Hence a face centred design was realized at that point, changing the extreme to the lower limit with a pH of 3.3. From the suggested 20 experiments 13 were chosen by a backward algorithm showing a D-efficiency of 65.29%. The results are displayed in main effects diagrams and in so-called Pareto charts. The optimization operations were conducted via statgraphics.
Main Effects Chart
The main effects diagrams display the main effects of the process parameters on the target parameters. The main effect is the effect of a process parameter on a target parameter averaging across the levels of the other process parameters and gives information about the overall effect of a parameter [24]. It is calculated as the difference of the mean values of two steps:
Here y1, y2 and y3 are the values of a target parameter at an upper level (220 °C, 390 min, pH 8) and y4, y5 and y6 are the values of a target parameter at a lower level (180 °C, 60 min, pH 3.3). In the main effects chart the average of a target parameter at every step (three steps for temperature, time and pH) are displayed. A second degree polynomial is used as a regression function, as it adapts to the data best.
In order to compute the interaction between two effects, following equation is used [24]:
where EA,B(+) refers to the effect of factor “A” at high level of factor “B” and EA,B(−) refers to factor “A” at low level of factor “B”. Due to the built-up of the design (Fig. 1) upper and lower levels, as mentioned before, were considered when calculating the main effect and the interactions. Existing optima at middle values are not considered, but are discussed in the text.
Pareto Chart
Within the Pareto chart the standardized effects are displayed according to their size, with the highest effect on top. The Pareto chart makes it very clear, which parameter influences the target parameter to a high degree and if it increases or decreases the target parameter. The standardized effect is computed by summing up all the main effects and the interactions and then set reference of one effect to the sum of all of the rest [24]:
Results and Discussion
Nutrient Recovery, Degree of Carbonization and Optimization Operation
Design of Experiment – Regression Functions
In S1 the regression functions of the target parameters carbon recovery, dry mass recovery, nitrogen recovery and phosphorus recovery are displayed. Also coefficients of determination of the regression functions are displayed. With R² values of 82.36 for carbon recovery, 91.23 for dry mass recovery, 96.90 for nitrogen recovery, 94.36 for phosphorus recovery and 96.92 for difference in O/C-ratio the quadratic regression functions approximate the data well.
Dry Mass Recovery
Dry mass recovery is defined as the dry mass of the residue from carbonization, separated with a sieve with a mesh size of 1 mm, divided by the total dry mass input into the reactor (feed) using a mesh size for sieving of 1 mm as well. The rather big mesh size reflects the wish to minimize extra energy input for separation, when hydrothermal carbonization is applied. The feed consists of a solid phase and a phase that is solved in the overhead liquid and was dried as a whole in order to compare it to the hydrochar.
In Fig. 2a the main effects of temperature, time and pH on dry mass recovery are displayed. Compared to previous works it can be seen, that dry mass recovery is generally 15–20 wt% lower at the same process conditions [17]. This is due to the big mash size of the sieve. Coarse sieve separation is energetically advantageous. Still, considerable amounts of dry mass and carbon are lost in the process water. It can be seen that with higher temperature und longer carbonization time the dry mass recovery generally decreases. At 180 and 220 °C the main effects chart shows values of 35 and 25 wt%, respectively. Concerning time the value ranges from 31 wt% at 60 min and 29 wt% at 390 min. The temperature curve is steeper than the time curve, indicating that with rising temperature the dry mass is affected more than if only the carbonization time is prolonged. That is reasonable, due to the changed properties of water that favours biomass degradation at higher temperature [25]. With rising pH the dry mass recovery also increases. It varies in a broad range between 21 wt% at pH 3.3 and 39 wt% at pH 8, showing a high influence on dry mass recovery. It should be kept in mind, that a decrease in pH means an increase in added citric acid. Due to the high amount of citric acid it is seen as feed material. As it is an organic acid it is solved in the process water. Nevertheless, as organic acids are important end products of hydrothermal carbonization, the added citric acid can have an impact on all the target parameters and as mentioned above has to be seen as part of the feed.
Main effects of process parameters temperature, time and pH on the target parameter dry mass recovery (a), carbon recovery (b), and difference in O/C-ratios (c). The points represent the mean values of the target parameters at a certain value of a process parameter. The regression line shows the general trend of a process parameter influencing a target parameter
In the Pareto Chart (Fig. 3a) the process parameters are listed according to their standardized effect. It can be observed that the pH has the highest effect, followed by the effect of temperature and the interaction of time and temperature. The effect of the pH is positive, meaning dry mass increases with increasing pH. The effect of temperature and also temperature–time interaction is negative. A negative interaction indicates, that the combination of a high and a low process parameter influences the target parameter. At pH 8 carbonization at 180 °C and 390 min as well as at 220 °C and 60 min results in the highest dry mass recoveries. This can be observed in Table 2, experiments 1–4. If the pH is decreased to a pH of 3.3, hydrolysis is reinforced with rising temperature. That can be seen in Table 2, experiments 9, 10 and 12, 13. Still, dry mass recovery is higher for the combination of low temperature (180 °C) and high carbonization time (390 min) than compared to the combination of low temperature (180 °C) and short carbonization time (60 min). Correspondingly, the combination of high temperature (220 °C) and short carbonization time (60 min) results in a higher dry mass recovery than the combination of high temperature (220 °C) and long carbonization time (390 min).
Carbon Recovery
Carbon recovery varies in a broad range between the values of 24 and 65 wt% (see Table 2). At a pH of 5.5, carbon recoveries at 165 and 230 °C show the same values. That interesting as the carbonization time was 225 min for both experiments (see Table 2). The highest carbon yield is reached at 180 °C, 390 min and a pH of 8.
With harsher conditions (lower pH, higher temperature) carbon recovery is decreased. The effects of temperature and pH on carbon recovery (see Fig. 2b) show the same trend as on dry mass recovery (see Fig. 2a). That can be expected, as the dry mass of the hydrochar decreases when carbon is set free. The main effects chart shows a value of 47 and 36 wt% at 180 and 220 °C, respectively.
The effect of time shows a maximum for carbon recovery, meaning that first other elements, like oxygen, are set free from the solid material to a higher degree than carbon is set free. This way the carbon content is increased until a maximum is reached. If the time-curves for dry mass recovery and carbon recovery are compared (see Fig. 2a, b), it can be seen that while the dry mass decreases with time, the carbon tends to increase. Again it can be seen that carbonization occurs, as carbon is enriched by the fact the other elements leave the biomass as they are solved in the process water.
The standardised Pareto-chart (see Fig. 3b) shows, that the pH has the highest effect on carbon recovery. The time–temperature interaction (AB) has the second highest effect on carbon recovery. As for dry mass recovery its effect is negative. The carbon recovery that results from long carbonization times (390 min) decreases with higher temperature. It is raised with temperature if the carbonization time is short (60 min, see Table 2). As for dry mass recovery, also the effect of temperature is high.
O/C-ratio Between Feed and Hydrochar (Degree of Carbonization)
As shown in Table 2 the difference of O/C-ratio between feed and hydrochar is between 0.10 and 0.76. In the main effects chart (see Fig. 2c) it is shown that at a temperature of 180 °C the difference in O/C-ratios is 0.43. It reaches a value of 0.47 at 220 °C, hence the temperature has a minor effect in the temperature range between 180 and 220 °C. At a carbonization time of 60 min the difference in O/C-ratios shows a value of 0.41 and reaches a value of 0.50 at 390 min. At a pH of 3 the difference in O/C-ratios is 0.67 and at pH 8 it has a value of 0.21. The amount of citric acid added was also included in computing the O/C-ratio. Consequently, the high influence of the pH may be due to the high O/C-ratio in the feed, caused by the added citric acid. Nevertheless, it is assumed that a lower pH favours hydrolysis. As oxygen-rich compounds diffuse into the process water preferentially, increased carbonization is expected at lower pH. Reza et al. carbonized wheat straw at 200 °C and 6 h carbonization time at pH from 2 to 12. They showed that the O/C- ratios in the hydrochars are always lower at a lower pH, hence the difference in O/C-ratios between feed and hydrochar is higher at lower pH [26].
The Pareto chart (see Fig. 3c) shows that pH has by far the highest effect, followed by time and the interaction of time and temperature. Again, the interaction effect of time–temperature is negative. It can be seen that the effect of temperature is rather small. This is because higher temperature favours hydrolysis of oxygen rich compounds but also of carbon rich compounds. The effect of the pH seems to be tremendously high compared to the effects of the other process parameters. Still it has to be kept in mind that with the addition of citric acid, also additional carbon and oxygen are added to feed and hydrochar.
Nitrogen Recovery
Table 2 shows that nitrogen recovery varies in a range between 10 and 60 wt%. The main effects chart (see Fig. 4a) shows that while at a temperature of 180 °C 40 wt% of the nitrogen stays in the hydrochar, the nitrogen recovery is considerably lower at a temperature of 220 °C with a value of about 21 wt%. This trend can be explained by the higher degree of hydrolysis at higher temperature. With higher temperature, more biomass is degraded, therefore more organic compounds containing nitrogen are in the process water or in the process gas. The same trend can be witnessed concerning pH. With higher pH, the nitrogen content is lower. It was expected, that at a pH of 3.3 more nitrogen-containing compounds are solved in the process water than at a pH of 8. The high amount of nitrogen at low pH is due to the equilibrium of nitrogen as its form as soluble ammonium and free ammonia. The equilibrium is dependent on temperature and pH. At higher temperatures and alkaline pH, more nitrogen is present in the form of ammonia, which is volatile [27]. When drying the hydrochar at temperature after carbonization, nitrogen leaves the char in its form of ammonia. That shows, that post-treatment can still alter composition and nutrient content of hydrochar considerably. In fact, if anaerobic digestate is applied on fields as a fertilizer, loosing nitrogen in der form of ammonia is already an issue due its alkaline pH [28].
Main effects of process parameters temperature, time and pH on the target parameter nitrogen recovery (a) and phosphorus recovery (b). The points represent the mean values of the target parameters at a certain value of a process parameter. The regression line shows the general trend of a process parameter influencing a target parameter
Nitrogen recovery is high at a pH of 3.3 with a value of around 40 wt% while it is considerably lower at a pH of 8 with a value of around 21 wt%. Ekpo et al. investigated the influence of pH on the nitrogen and phosphorus extraction of swine manure into the process water during hydrothermal carbonization. They added 0.1 M organic acids as well as 0.1 M sulfuric acid and water to the dry swine manure in order to compare. They report that extraction of nitrogen into the process water was not significantly affected by pH and was between 25 and 29% extraction at 120 °C, between 44 and 49% at 180 °C and between 47 and 50% at 200 °C although the pH was around 4 after carbonization [29]. The time-curve for nitrogen recovery shows an optimum. The values at 60 and 390 min are between 30 and 31 wt%, hence are very close. As the effect is computed as the difference between the highest and the lowest value, the effect is low. The value in between at 230 min has an optimum at around 38 wt% nitrogen recovery. In the Pareto chart (see Fig. 5a) it can be seen that temperature has the highest effect on nitrogen recovery followed by the effect of pH. The effect of time is very small, which is due to the fact that it seems to have an optimum.
Phosphorus Recovery
Phosphorus recovery varies in a range between 10 and 57 wt% (see Table 2). In the main effects chart (see Fig. 4b) phosphorus recovery shows an optimum for temperature, time and pH. It is very likely that this is due to the strong effect of the pH. Most the measurement points at the middle values are at a pH of 5.5, which is the optimal pH for phosphorus recovery (see Fig. 1). The main effect is the effect of a process parameter on a target parameter averaging across the levels of the other process parameters. Therefore, if there is a very strong effect of one process parameters it also affects the main effects of the other parameters. It can be seen that pH has the highest effect (see Fig. 5b). At a low pH of 3.3 the phosphorus recovery has a value of about 22 wt%, while at a higher pH of 8 it has a value of around 39 wt%. That can be expected, because more feed biomass is broken down at a lower pH, hence also less phosphorus is recovered in the hydrochar. Also, the effect of the charge of the biomass compound containing phosphorus should be kept in mind. At a higher pH, the compound is uncharged, hence stays in the hydrochar and is not dissolved in the process water. The same effect of the pH during hydrothermal carbonization on phosphorus recovery was shown by Ekpo et al. at 170, 200 and 250 °C but the adverse effect was reported at 120 °C [29]. It is interesting that phosphorus recovery has an optimum regarding temperature. At 180 °C the phosphorus recovery is 31 wt%, at 220 °C 28 wt%. At the middle value of 200 °C the phosphorus recovery has a maximum value of 44 wt%. It can be expected that less phosphorus is recovered in the hydrochar at higher temperatures, because biomass is degraded to a higher extent. However, this is not the case. Ekpo et al. carbonized swine manure from 120 to 250 °C and they report that less phosphorus was extracted into the process water at higher temperature [29]. Sumida et al. carbonized sewage sludge hydrothermally and report that with higher temperature more phosphorus is solved into the process water [30]. Phosphorus recovery also shows an optimum concerning time. At a carbonization time of 60 min it has a value of 20 wt%, at 225 min a value of 41 wt% and at 390 min a value of 30 wt%. The Pareto chart shows that the pH has the highest effect on phosphorus recovery and contrary to nitrogen recovery it is negative. In Fig. 5b it can also be seen that time and temperature have the lowest effects. This is because they show an optimum.
Optimization Operations
Figure 6 shows two contour plots. They illustrate to what degree the process parameters temperature, time and pH affect the recovery for dry mass, carbon, phosphorus and nitrogen as well as the difference in O/C-ratio. The highest possible recovery for dry mass in the used model is 50.34 wt% with a temperature of 165 °C, 500 min of carbonization time and a pH of 6.8 (see Fig. 6a; Table 3). Whereas the area in which a recovery of over 50 wt% is possible is rather small, a dry mass recovery of over 40 wt% can be reached in a wider range concerning temperature, time and pH. The optimal temperature is, with a value of 165 °C, rather low. That is due to the fact that the highest dry mass is recovered when no or little carbonization reactions occur, hence no hydrolysis occurs.
Concerning carbon recovery, the highest possible value in the used model is 67.45 wt% at a temperature of 165 °C, 500 min and a pH of 6.4 (see Fig. 6b; Table 3). The higher values for carbon recovery compared to the values of dry mass recovery at the same process parameters shows that carbonization occurs due to other elements than carbon being depleted from the char.
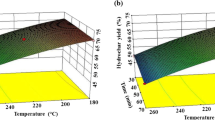
The highest value for nitrogen recovery within the model is 67.77 wt% at 165 °C, 335 min and a pH of 3.3 (see Fig. 6c; Table 3). However, as the proteins present in the anaerobic digestate were degraded during hydrothermal carbonization (see Chap. 2. Chemical structure of the hydrochars) it was expected that at a carbonization pH of 3.3 the nitrogen containing compounds are present in the process water. The highest value for phosphorus recovery within the model is 63.43 wt% at 165 °C, 500 min and a pH of 8 (see Fig. 6d; Table 3). It can be seen that an enrichment either in phosphorus or in nitrogen is possible via adjustment of the pH. If the goal is to recover nutrients and also dry mass, low temperatures at around 165 °C are the optimal temperatures. Nevertheless, carbonization does not occur to a high degree at these temperatures. When it comes to carbonization (difference of O/C-ratio between feed and hydrochar, Fig. 6e; Table 3) the favourable process conditions are opposite to the process conditions favouring nutrient recycling concerning temperature and pH. The optimal process conditions are 230 °C, 500 min of carbonization time and a pH of 3.3. Especially pH and temperature are crucial for nutrient and dry mass recovery.
Figure 7 shows to which extent the desired hydrochar properties can be reached by altering the process parameters to the optimal values. If the goal is to recycle carbon and nitrogen in order to produce a soil amendment that can be sold as a product on the one hand and also to avoid overfertilization close to biogas plants, the optimal process conditions are 165 °C, 500 min and a pH of 5.3 (see Fig. 7a; Table 3). These are the conditions if the goal is to maximize nitrogen and carbon recovery. The predicted carbon recovery within this model is 66 wt% and the predicted nitrogen recovery is 54 wt%. Especially concerning nitrogen content in the char the equilibrium between solved ammonium and volatile ammonia should be considered. The desired recovery values of 70 wt% for carbon content and of 60 wt% for nitrogen content cannot be reached, but are rather close. If the aim is to burn the resulting hydrochar to use it as solid fuel, the difference in O/C-ratios between feed material and hydrochar should be maximized as that also maximizes the heating value and the dry mass recovery should be high. The optimal conditions if difference between O/C-ratios and dry mass recovery should be maximized are a temperature of 165 °C, a carbonization time of 500 min and a pH of 3.5 (see Fig. 7b; Table 3). At these conditions the difference of O/C-ratios has a value of 0.8 and a dry mass recovery of 36 wt% can be reached according to the model. Nevertheless, Fig. 7b shows that over a broad pH range, the combination of high temperature and short carbonization time and low temperature and long carbonization time lead to a good result, if the ai is to use hydrochar from biogas slurry as solid fuel.
Optimized process condition regarding carbon recovery and nitrogen recovery (a) and regarding dry mass recovery and difference in O/C-rations between feed and hydrochar (b). The colours indicate in how far the desired target parameters are fulfilled (see Table 1). (Color figure online)
Chemical Structure of Hydrochars
To gain some information about the structural composition of the hydrochars as well as information about structural changes during different treatment conditions samples were analyzed by solid state NMR spectroscopy. As the feed material consisted of food and agricultural wastes the main composition should consist of cellulose, hemicelluloses, lignins but also lipids and proteins, respectively. 13C solid state NMR of feed material (Fig. 8) showed a strong signal at δ ca. 105 ppm - characteristic for the anomeric C-1 of natural cellulose – and furthermore the typical signal pattern for cellulose carbons 2–6 in the region of δ = 90–60 ppm. The presence of lignin was characterised by the methoxyl group resonance at δ ca. 55 ppm and broad signals in the aromatic region between δ 150 and 100 ppm. Signals around δ = 30 ppm accompanied by a sharp resonance of a double bond at 130 ppm revealed the presence of long aliphatic chains typical of saturated and unsaturated fatty acids.
In the 15N solid state NMR spectra a broad peak at around −260 ppm, characteristic for amide bonds, confirmed the presence of proteins.
The fate of cellulose during hydrothermal treatment under neutral pH conditions is assumed to be a direct conversion into polyaromatic compounds at temperatures from 200 °C upwards [31]. The onset temperature of this conversion is lowered with increasing presence of impurities and accompanying components in the cellulose matrix. In the case of our studies at mild alkaline conditions of pH 8 and a temperature of 180 °C (see Table 2 experiment 1) no degradation of cellulose were detected compared to the feed (see Fig. 9a showing the feed and b). Degradation of cellulose slightly increased with decreasing pH values, since cleavage of the glycosidic bond is an acid-catalyzed process. In Fig. 9c, showing experiment nr. 8 (see Table 2) a decrease in cellulose can be witnessed, which is expected due to a high carbonization temperature of 230 °C and a pH of 5.5. That the pH alone is not responsible for degradation of cellulose can be seen in Fig. 9d, where still high amounts of cellulose are present. During this experiment (see Table 2, experiment 5) the pH of 5.5 was lower compared to the feed material, still carbonization temperature was very low with a value of 165 °C. Also at a low pH of 3.3, long reaction time of 390 min and low temperature of 180 °C cellulose was degraded to a little degree (see Fig. 9e; Table 2, experiment 10). Harsh conditions of a pH of 3.3, a temperature of 220 °C and a carbonization time of 390 min decreased the cellulose content considerably (see Fig. 9f; Table 2, experiment 13), but still small amounts of cellulose were present in the hydrochar. Unfortunately, no NMR measurements could be made of a sample carbonized at pH 8 and higher temperatures. However, Reza et al. carbonized digestate from wheat straw without changing the pH and they report a reduction of cellulose in the hydrochar due to a relative increase of aromatic and aliphatic carbon atoms with higher temperature. They also state that no considerable degradation of cellulose happened at 180 °C, while degradation seems to start at 220 °C and aromatization is increased at this temperature with longer carbonization time [32]. It can be seen that higher acidic conditions combined with high temperature increased the hydrolysis rate of cellulose to smaller chains and finally glucose units which are better carbonized via furanoid intermediates as described by Coronella et al. [11].
Contrary to the behaviour of cellulose that was largely consumed at high temperatures, lipids did not show high reactivity which is documented by the sharp and prominent double bond signal at δ = 130 ppm still detected also at relatively harsh conditions. Obviously oxidation and degradation of the fatty acids need more drastic conditions preferably higher pH values or higher oxygen concentrations to attack the double bonds. This is in line with general chemical knowledge: since cellulose degradation involves heterolytic reaction steps, such as hydrolyses, elimination reactions and condensations, the cleavage of the largely inert aliphatic chains of fatty acids requires radical processes (homolytic C–C bond cleavages) that require higher activation energies.
In general, by comparison of the 13C spectra of the different hydrochars the highest carbonization rate was found at low pH values, high reaction temperatures and long reaction times observable as broad, intense signals in the aromatic region from δ = 115–150 ppm. Comparing plot C and plot F in Fig. 9 the influence of pH and carbonization time can be witnessed, as the signal in the aromatic region is stronger in plot F (220 °C, 390 min, pH 3.3) that in plot C (230 °C, 225 min, pH 5.5). Figure 10 shows the reaction product at a temperature of 245 °C, a reaction time of 300 min and a pH of 3.3 compared to the feed (see Table 2, experiment 14). For this experiment sulfuric acid was used to alter the pH, hence a high degree of carbonization was expected. In this case cellulose was fully carbonized, also lignins and aliphatic compounds are partially degraded and the carboxylic groups were largely diminished, probably by decarboxylation. For this experiment a different anaerobic digestate was used than for the other experiment. This digestate is derived from different agricultural wastes and plants, planted as renewable resources for biogas production. High amounts of cellulose are present in this anaerobic digestate, whereas the amount of lipids is very low, as can be expected.
We also performed 15N solid state NMR spectroscopy to get some insight to the fate of proteins. As can be seen in Fig. 11, besides the amide derived resonances an additional peak at δ = −205 ppm is observed which suggests not only the obvious denaturation of proteins but indicates partial degradation by formation of heteroaromatic derivatives. The underlying processes are reductive cyclizations.
Conclusion
The results show that temperature and pH are important process parameters for nitrogen and phosphorus recovery. The combination of a high degree of nutrient recovery within the hydrochar and a high degree of carbonization is not possible, as nutrients remain in the hydrochar at low temperatures more and a high degree of carbonization requires a high temperature. Thus, a useful approach could be to extract the nutrients in the process water and carbonize the residual biomass to a high degree. If the nutrients can be recovered from the process water, as it is the case for phosphorus recovery via the struvite process, the resulting hydrochar can either be enriched with nutrients again or used as a solid fuel. Optimized temperature for nutrient recovery is 165 °C, a temperature where carbonization is not promoted very much. The optimal pH for nutrient recovery was expected to be high. This is the case for phosphorus recovery with a value of 8. Nitrogen recovery showed the opposite trend. This can be explained by the pH and temperature dependent equilibrium of solved ammonium and free ammonia. With higher pH and higher temperature, the equilibrium is shifted towards free ammonia that is volatile. Concerning carbonization, the time–temperature effect seems to be important. Similar degrees of carbonization can be reached at short carbonization time and high temperature as well as at long carbonization time and low temperature. Nevertheless, the best results are obtained at a pH of 3 and a temperature of 230 °C. In general, a long carbonization time of 500 min was given as the optimized value. However, as the influence of time is small in general, therefore this value can be reduced in industrial application of HTC. Applying a coarse sieve to separate liquid and solid phase resulted in reduced dry mass recoveries. The recoveries are 15–20 wt% lower than reported in previous works for the same reaction conditions. Coarse sieve separation is energetically advantageous, still considerable amounts of dry mass are left in the process water. It was shown that enrichment of carbon in the hydrochar occurs due to diffusion of oxygen rich compounds into the process water at temperatures up to 220 °C. Changes in the chemical structure of the hydrochar begin at 220 °C, while a low pH improves carbonization. It was shown that cellulose is present in the anaerobic digestate and can be fully degraded at high temperatures and a low pH. Additionally, it was shown that a high degree of aromatization could be reached at severe reaction conditions.
References
Foley, J.A., Ramankutty, N., Brauman, K.A., Cassidy, E.S., Gerber, J.S., Johnston, M., et al.: Solutions for a cultivated planet. Nature 478(7369), 337–342 (2011)
Cordell, D., Drangert, J.O., White, S.: The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 19(2), 292–305 (2009)
Peñuelas, J., Poulter, B., Sardans, J., Ciais, P., van der Velde, M., Bopp, L., et al.: Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. http://www.nature.com/doifinder/10.1038/ncomms3934. Accessed 25 July 2016
Geupel, M., Frommer, J.: Reactive nitrogen in Germany. Causes and effects - measures and recommendations. Umweltbundesamt, Dessau-Roßlau (2014)
Ribbe, L., Freibauer, A., Güthler, W., Heißenhuber, A., Hülsbergen, K.J., Krug, A., et al.: Biogaserzeugung und -nutzung: Ökologische Leitplanken für die Zukunft. Kommission Landwirtschaft beim Umweltbundesamt, Dessau-Roßlau (2013)
Wang, L., Zhang, L., Li, A.: Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: influence of operating conditions and the process energetics. Water Res. 65, 85–97 (2014)
Reza, T., Freitas, A., Yang, X., Hiibel, S., Lin, H., Coronella, C.J.: Hydrothermal carbonization (HTC) of cow manure: carbon and nitrogen distributions in HTC products. Environ. Prog. Sustain. Energy. (2016). doi: 10.1002/ep.12312
Sun, Y., Gao, B., Yao, Y., Fang, J., Zhang, M., Zhou, Y., et al.: Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 240, 574–578 (2014)
Bargmann, I., Rillig, M.C., Buss, W., Kruse, A., Kuecke, M.: Hydrochar and biochar effects on Germination of Spring Barley. J. Agron. Crop. Sci. 199(5), 360–373 (2013)
Peterson, A.A., Vogel, F., Lachance, R.P., Fröling, M., Antal, M.J., Tester, J.W.: Thermochemicalbiofuel production in hydrothermal media: a review of sub- and supercritical watertechnologies. Energy. Environ. Sci. 1(1), 32 (2008)
Libra, J.A., Ro, K.S., Kammann, C., Funke, A., Berge, N.D., Neubauer, Y., et al.: Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels. 2(1):71–106 (2011)
Pavlovič, I., Knez, Ž., Škerget, M.: Hydrothermal reactions of agricultural and food processing wastes in sub- and supercritical water: a review of fundamentals, mechanisms, and state of research. J. Agric. Food Chem. 61(34), 8003–8025 (2013)
Akizuki, M., Fujii, T., Hayashi, R., Oshima, Y.: Effects of water on reactions for waste treatment, organic synthesis, and bio-refinery in sub- and supercritical water. J. Biosci. Bioeng. 117(1), 10–18 (2014)
Siskin, M., Katritzky, A.R.: Reactivity of organic compounds in superheated water: general background. Chem. Rev. 101(4), 825–836 (2001)
Coronella, C.J., Lynam, J.G., Reza, M.T., Uddin, M.H.: Hydrothermal carbonization of lignocellulosic biomass. In: Jin, F, (ed.) Application of hydrothermal reactions to biomass conversion [Internet], pp. 275–311. Springer, Berlin. http://link.springer.com/10.1007/978-3-642-54458-3_12. Accessed 12 Jan 2016
Spokas, K.A..: Review of the stability of biochar in soils: predictability of O:C molar ratios. Carbon Manag. 1(2), 289–303 (2010)
Funke, A., Mumme, J., Koon, M., Diakité, M.: Cascaded production of biogas and hydrochar from wheat straw: Energetic potential and recovery of carbon and plant nutrients. Biomass Bioenergy 58, 229–237 (2013)
Mumme, J., Eckervogt, L., Pielert, J., Diakité, M., Rupp, F., Kern, J.: Hydrothermal carbonization of anaerobically digested maize silage. Bioresour. Technol. 102(19), 9255–9260 (2011)
Escala, M., Zumbühl, T., Koller, C., Junge, R., Krebs, R.: Hydrothermal carbonization as an energy-efficient alternative to established drying technologies for sewage sludge: a feasibility study on a laboratory scale. Energy Fuels 27(1),454–460 (2013)
Heilmann, S.M., Molde, J.S., Timler, J.G., Wood, B.M., Mikula, A.L., Vozhdayev, G.V., et al.: Phosphorus reclamation through hydrothermal carbonization of animal manures. Environ. Sci. Technol. 48(17), 10323–10329 (2014)
Du, Z., Hu, B., Shi, A., Ma, X., Cheng, Y., Chen, P., et al.: Cultivation of a microalga Chlorella vulgaris using recycled aqueous phase nutrients from hydrothermal carbonization process. Bioresour. Technol. 126, 354–357 (2012)
Danso-Boateng, E., Holdich, R.G., Wheatley, A.D., Martin, S.J., Shama, G.: Hydrothermal carbonization of primary sewage sludge and synthetic faeces: effect of reaction temperature and time on filterability. Environ. Prog. Sustain. Energy 34(5), 1279–1290 (2015)
Sabio, E., Álvarez-Murillo, A., Román, S., Ledesma, B.: Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: influence of the processing variables. Waste Manag. 47, 122–132 (2016)
Antony, J.: Design of experiments for engineers and scientists. Butterworth Heinemann, Burlington (2008)
Funke, A., Ziegler, F.: Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod Biorefin. 4,160–177 (2016)
Reza, M.T., Rottler, E., Herklotz, L., Wirth, B.: Hydrothermal carbonization (HTC) of wheat straw: influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 182, 336–344 (2015)
Monlau, F., Sambusiti, C., Ficara, E., Aboulkas, A., Barakat, A., Carrère, H.: New opportunities for agricultural digestate valorization: current situation and perspectives. Energy Environ. Sci. 8(9), 2600–2621 (2015)
Nkoa, R.: Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agron Sustain. Dev. 34(2), 473–492 (2014)
Ekpo, U., Ross, A.B., Camargo-Valero, M.A., Fletcher, L.A.: Influence of pH on hydrothermal treatment of swine manure: impact on extraction of nitrogen and phosphorus in process water. Bioresour. Technol. 214, 637–644 (2016)
Sun, X.H., Sumida, H., Yoshikawa, K.: Effects of hydrothermal process on the nutrient release of sewage sludge. Int. J. Waste Resour. http://www.omicsonline.com/openaccess/effects-of-hydrothermal-process-on-the-nutrient-release-of-sewage-sludge-2252-5211.1000124.php?aid=21378. Accessed 12 Sept 2016
Falco, C., Baccile, N., Titirici, M-M.: Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 13(11), 3273 (2011)
Reza, M.T., Mumme, J., Ebert, A.: Characterization of hydrochar obtained from hydrothermal carbonization of wheat straw digestate. Biomass Convers. Biorefinery 5(4), 425–435 (2015)
Acknowledgements
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). The authors would like to thank wpa Beratende Ingenieure for supply of the feed material. The research conducted was supported by grants commissioned from the Austrian Research Promoting Agency FFG (Project FERTI-MINE, Project Nr. 844744) within the programme Research Studios Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Stutzenstein, P., Bacher, M., Rosenau, T. et al. Optimization of Nutrient and Carbon Recovery from Anaerobic Digestate via Hydrothermal Carbonization and Investigation of the Influence of the Process Parameters. Waste Biomass Valor 9, 1303–1318 (2018). https://doi.org/10.1007/s12649-017-9902-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9902-4