Abstract
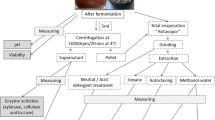
The present study was undertaken to evaluate the saccharification, followed by bioethanol production by an actinobacterium Streptomyces variabilis (MAB3) isolated from the sediment sample of mangrove environment using Modified Yeast extract—Peptone medium substituted with different concentrations of xylanase (50 to 400 IU) pretreated various agro-residues like vegetable, banana, mango, sugarcane bagasse and sugarcane juice through fermentation process. Among the tested residues, sugarcane juice substituted medium pretreated with 200.0 IU concentration of xylanase exhibited maximum level of reducing sugar (50.19 mg/g), degree of saccharification (59.49%) as well as bioethanol (4.69 g/L) production. Further the organism expressed highest saccharification yield and bioethanol production at the optimized cultural conditions of pH 6, temperature 30 °C, inoculum size 2.5% and incubation time 72 h substituted with 2.5% dextrose and 2.0% urea, respectively as carbon and nitrogen sources. The concentration of produced bioethanol (82.26%) from the optimized medium was determined through HPLC analysis. Finally the bioethanol was evaluated through FT-IR and GC-MS analysis. The basic functional ethanol groups (O–H and C–O) from the optimized medium were denoted in both FT-IR and GC-MS analysis at the wavenumber of 3272.22 and 1077.15 cm−1 with the retention time of 2.60 min, respectively. Based on the results, it could be confirmed that the selected isolate is a potent strain and it can able to hydrolyze the pretreated agro-residues and also it can able to convert the pretreated agro residues into economically important byproduct like bioethanol.
Graphical Abstract







Similar content being viewed by others
References
Buaban, B., Inoue, H., Yano, S., Tanapongpipat, S., Rengpipat, S., Eurwilaichitr, L.: Bioethanol production from ball milled bagasse using an on-site produced fungal enzyme cocktail and xylose-fermenting Pichia stipitis. J. Biosci. Bioeng. 110, 18–25 (2010)
Girio, F.M., Fonseca, C., Carvalheiro, F., Duarte, L.C., Marques, S., Bogel-Lukasik, R.: Hemicelluloses for fuel ethanol: a review. Bioresour. Technol. 101, 4775–4800 (2010)
Chandrakant, P., Bisaria, V.S.: Simultaneous bioconversion of cellulose and hemicelluloses to ethanol. Critc. Rev. Biotechnol. 18, 295–331 (1998)
Lee, S.J., Kim, S.B., Kang, S.W., Han, S.O., Park, C., Kim, S.W.: Effect of crude glycerol-derived inhibitors on ethanol production by Enterobacter aerogenes. Bioprocess Biosys. Eng. 35, 85–92 (2010)
Howard, R.L., Abotsi, E., Jansen van Rensburg, E.L., Howard, S.: Cellulase production from fungal isolate Fusarium sp. Afr. J. Biotechnol. 2, 602–619 (2003)
Wyman, C.E., Dale, B.E., Elander, R.T., Holtzapple, M., Ladisch, M.R., Lee, Y.Y.: Coordinated development of leading biomass pretreatment technologies. Biotechnol. Prog. 96, 1959–1966 (2005)
Lynd, L.R., Weimer, P.J., Van Zyl, W.H., Pretorius, I.S.: Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66, 506–577 (2002)
Chang, V.C., Holzapple, M.T.: Fundamental factors affecting biomass enzymatic reactivity. Appl. Biochem. Biotechnol. 5, 37–47 (2000)
Walia, B.S., Tan, L.U., Saddler, J.N.: Purification and characterization of an alkaline xylanase from Streptomyces viridosporus T7A. Microbiol. Rev. 52, 305–317 (2012)
Beg, Q.K., Bhushan, B., Kapoor, M., Moondal, G.S.: Enhanced production of a thermostable xylanase from Streptomyces sp. QG-11-3 and its application in bioleaching of eucalyptus kraft pulp. Enzy. Microbial. Technol. 27, 459–466 (2000)
Sriyapai, D.H., Alves, L., Ribeiro, S., Amaral-Collac, O.M.T: Overproduction and biological activity of prodigiosin-like pigments from recombinant fusant of endophytic marine Streptomyces species. Antonie Van Leeuwenhoek. 102, 719–734 (2011)
Ragauskas, L.R., Gomes, R.C., Manfio, G.P., Alviano, C.S., Linhares, L.F.: Production and partial characterization of xylanase from Streptomyces sp. strain AMT-3 isolated from Brazilian cerrado soil. Enzy. Microbial Technol. 31, 549–555 (1994)
Zhou, S., Abotsi, E., Ingram, L.O.: Synergistic hydrolysis of carboxymethyl cellulose and acid swollen cellulose by two endoglucanase (Cel Z and Cel Y) from Erwinia chrysantheme. J. Bacteriol. 182, 5676–5682 (2010)
Ninawe, S., Kuhad, R.C.: Use of xylan-rich cost effective agro-residues in the production of xylanase by Streptomyces cyaneus SN32. J. Appl. Microbiol. 99, 1141–1148 (2006)
Bajaj, B.K., Singh, S.M.: Studies on an alkali-thermostable xylanase from Aspergillus fumigatus MA28. Biotechnology 1, 161–171 (2010)
APHA: Standard Methods for the Examination of Water/Waste Water. APHAAWWA-WPCF, Washington, DC (1995)
ASTM: Annual Book of American Society for Testing and Materials Standard, vol. 1527. ASTM, Philadelphia (1979)
RadhaKrishnan, M., Balaji, S., Balagurunadhan, R.: Thermotolerant actinomycetes from the Himalayan Mountain antagonistic potential characterization and identification of selected strains. Malay. J. Appl. Biol. 36, 59–65 (2007)
Thenmozhi, R., Victoria, J.: Optimization and improvement of ethanol production by the microporation of organic wastes. Pelagia research library. Adv. Appl. Sci. Res. 4, 119–123 (2013)
Shiling, E.B., Gottlieb, D.: Methods for characterization of Streptomyces species. Int. J. Sys. Bacteriol. 16, 312–340 (1966)
Nathan, A., Jessica, M., Bernan, V., Dworkin, M., Sherman, D.H.: Isolation and characterization of novel marine derived actinomycete taxa rich in bioactive metabolites. Appl. Environ. Microbiol. 70, 7520–7529 (2004)
Thompson, J.D., Gibson, J.J., Plewniak Jeanmougin, F., Higgins, D.G.: The clustal windows interface: flexible strategies for multiple sequence alignment aided by quality analysis stools. Nucleic Acid Res. 24, 4876–4882 (1997)
Sanjivkumar, M., Silambarasan, T., Palavesam, A., Immanuel, G.: Biosynthesis, purification and characterization of β-1,4-xylanase from a novel mangrove associated actinobacterium Streptomyces olivaceus (MSU3) and its applications. Protein Expr. Purif. 130, 1–12 (2017)
Detroy, R.W., Cunningham, R.L., Bothast, R.J., Bagby, M.O.: Bioconversion of wheat straw cellulose/hemicelluloses to ethanol by Saccharomyces uvarum and Pachysolen tannophilus. Biotechnol. Bioeng. 24, 1105–1113 (1982)
Saritha, M., Arora, A., Singh, S., Nain, L.: Streptomyces griseorubens mediated delignification of paddy straw for improved enzymatic saccharification yields. Bioresour. Technol. 135, 12–17 (2013)
Ahmed, F., Rahmam, S.R., Gomes, D.J.: Saccharification of sugarcane bagasse by enzymatic treatment for bioethanol production. Malay. J. Microbiol. 8, 97–103 (2012)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Rao, R.S., Bhadra, B., Shivaji, S.: Isolation and characterization of ethanol producing yeasts from characterization of ethanol producing yeasts from fruits and tree barks. Lett. Appl. Microbiol. 47, 19–24 (2008)
Caputi, A., Ueda, M., Brown, T.: Spectrophotometric determination of ethanol in wine. Am. J. Environ. Vitic. 19, 160–165 (1968)
Hossain, M., Anantharaman, N., Das, M.: Bioethanol fermentation from untreated and pretreated lignocellulosic wheat straw using fungi Fusarium oxysporum. Ind. J. Chem. Technol. 19, 63–70 (2012)
Elumalai, S., Sakthivel, R.: GC-MS and FT-IR spectroscopic determination of fatty acid methyl ester of 16 fresh water microalgae, isolated from cement industries of TamilNadu, India. J. Algal Biomass Utn. 4, 50–69 (2013)
Dizhbite, T., Telysheva, G., Dobele, G., Arshanitsa, A., Kampars, V.: Py-GC/MS for characterization of non-hydrolyzed residues from bioethanol production from softwood. J. Analy. Appl. Pyrol. 90, 126–132 (2011)
Lineweaver, H., Burk, D.: The determination of enzyme dissociation constants. J. Am. Chem. Soc. 57, 685 (1934)
Jensen, P., Dwight, R., Fenical, A.: The distribution of actinomycetes in near-shore tropical marine sediments. Appl. Environ. Microbiol. 57, 1102–1108 (1991)
Williams, P.G.: Planning for chemical gold: marine bacteria as a source of new therapeutics. Trends Biotechnol. 27, 45–52 (2009)
Nithya, B., Ponmurugan, P., Fredimoses, M.: 16 S rRNA phylogenetic analysis of actinomycetes isolated from Eastern Ghats and marine mangrove associated with antibacterial and anticancerous activities. Afr. J. Biotechnol. 11, 12379–12388 (2012)
Remya, M., Vijayakumar, R.: Isolation and characterization of marine antagonistic actinomycetes from west coast of india. Medicine Biol. 15, 13–19 (2008)
Latif, F., Rajoka, M.: Production of ethanol and xylitol from corn cobs by yeasts. Bioresour. Technol. 77, 57–63 (2001)
Goodfellow, M., Willam, S.T., Mordarski, M.: Actinomycetes in biotechnology, Academic press, London (1988)
Rifaat, H.M., Awad, A.H., Gebreel, H.M.: Production of xylanase by Streptomyces sp. and their bleaching effect on rice straw pulp. Appl. Ecol. Environ. Res. 2, 45–51 (2004)
Anderson, A.S., Wellington, E.M.H: The taxonomy of Streptomyces and related genera. Int. J. Sys. Evol. Microbiol. 51, 797–814 (2001)
Clarkin, S.D., Clesceri, L.S.: Enzymatic hydrolysis and cellulose based ion-exchange powdered mixed resins. Appl. Microbiol. Biotechnol. 60, 485–488 (2002)
Zhang, X., Xu, C., Wang, H.: Pretreatment of bamboo residues with Coriolus versicolor for enzymatic hydrolysis. J. Biosci. Bioeng. 104, 149–151 (2007)
Bhattacharya, A., Ganguly, A., Subhabrata, D., Chatterjee, P., Apurba, D.: Fungal isolates from local environment: isolation, screening and application for the production of ethanol from water hyacinth. Int. J. Emer. Technol. Adv. Eng. 3, 58–65 (2013)
Zhu, J.Y., Pan, X.J., Wang, G.S., Gleisner, R.: Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour. Technol. 100, 2411–2417 (2009)
Kshirsagar, S.D., Saratale, G.D., Saratale, R.G., Oh, M.K.: An isolated Amycolatopsis sp. GDS for cellulase and xylanase production using agricultural waste biomass. J. Appl. Miocrobiol. 11, 136–141 (2016)
Ali, U.F., Ibrahim, Z.M., Isaac, G.S.: Ethanol and xylitol production from xylanase broth of Thermomyces lanuginosus grown on some lignocellulosic wastes using Candida tropicalis EMCC2. Life Sci. J. 10, 968–978 (2013)
Wan, C., Li, Y: Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour. Technol. 101, 6398–6403 (2010)
Patel, J., Onkarappa, R., Shobha, K.S.J: Comparative study of ethanol production from microbial pretreated agricultural residues. Appl. Sci. Environ. Manag. 11, 137–141 (2007)
Stalin, T., Sathya priya, B., Selvam, K.: Ecofriendly application of cellulase and xylanase producing marine Streptomyces clavuligerus as enhancer in biogas production from waste. Afr. J. Environ. Sci. Technol. 6, 258–262 (2012)
Belkacemi, K., Hamoudi, S.: Enzymatic hydrolysis of dissolved corn stalk hemicelluloses: reaction kinetics and modeling. J. Chem. Technol. Biotechnol. 78, 802–808 (2003)
Ray, R.R.: Saccharification of agro-wastes by endo-xylanase from Streptomyces sp OM09. Int. J. Life Sci. Biotechnol. Pharm. Res. 2, 165–170 (2013)
Huang, C., He, J., Li, X., Min, D., Yong, Q.: Facilitating the enzymatic saccharification of pulped bamboo residues by degrading the remained xylan and lignin carbohydrate complexes. Bioresour. Technol. 192, 471–477 (2015)
Christakopoulos, P.F., Koullas, D.P., Kekos, D., Koukios, E.G., Macris, B.J.: Direct ethanol conversion of pretreated straw by Fusarium oxysporum. Bioresour. Technol. 35, 297–300 (1989)
Mariam, I., Manzoor, K., Ali, S., Ikram-UI-Haq H.: Enhanced production of ethanol from free and immobilized Saccharomyces cerevisiae under stationary culture. Pak. J. Biotechnol. 41, 821–833 (2009)
Sindhu, V., Kanchana, C.N., Vasanthi, N.S., Ravikumar, R.: Design and development of novel bioreactor for the production of ethanol from low cost pretreated rice straw. IOSR J. Eng. 2, 1424–1428 (2012)
Bak, J.S., Ko, J.K., Choi, I.G., Park, Y.C.: Fungal pretreatment of lignocelluloses by Phanerochaete chrysosporium to produce ethanol from rice straw. Biotechnol. Bioeng. 104, 471–482 (2009)
Bhalt, N., Dharmesh, A., Thakor, P.: Production of xylanase by Aspergillus flavus (FPDN1) on pearl millet bran: optimization of culture conditions and application in bioethanol production. Int. J. Res. Chem. Environ. 2, 204–210 (2012)
Du, M., Huang, S., Wang, J.: The volatiles from fermentation product of Tuber formosanum. J. Forest. 4, 426–429 (2014)
Ahmad, F., Jameel, A.T., Kamarudin, M.H., Mel, M.: Study of growth kinetic and modeling of ethanol production by Saccharomyces cerevisiae. Afr. J. Biotechnol. 16, 18842–18846 (2011)
Shafaghat, H., Najafpour, G.D., Rezaei, P.S., Baei, M.S.: Ethanol production with natural carbon sources in batch and continuous fermentation using free and immobilized Saccharomyces cerevisiae. J. Sci. Ind. Res. 70, 162–164 (2011)
Imamoglu, E., Sukan, F.V.: Scale-up and kinetic modeling for bioethanol production. Bioresour. Technol. 144, 311–320 (2013)
Acknowledgements
The authors gratefully acknowledge the University Grants Commission (UGC), New Delhi, Govt. of India, for its financial support in the form of Special Assistance Programme (SAP) [UGC No. F.3–24/2012(SAPII) dtd October 2012].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanjivkumar, M., Brindhashini, A., Deivakumari, M. et al. Investigation on Saccharification and Bioethanol Production from Pretreated Agro-Residues Using a Mangrove Associated Actinobacterium Streptomyces variabilis (MAB3). Waste Biomass Valor 9, 969–984 (2018). https://doi.org/10.1007/s12649-017-9886-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9886-0