Abstract
Purpose
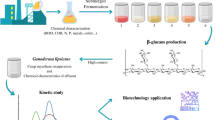
The aim of the present study was to investigate the feasibility of polysaccharides production by selected basidiomycetes in submerged culture. Olive mill wastewater (OMWW) was also tested as a potential substrate for polysaccharides production by mushroom strains, focusing on the simultaneous degradation and valorization of the waste material.
Methods
The tested strains were grown in two different substrates, and after biomass harvesting, polysaccharides were isolated using two different methods. The extracellular polysaccharides were isolated from the culture broth, with ethanol precipitation. The isolated fractions were partially characterized with FT-IR spectroscopy.
Results
All three strains performed well in both substrates. Maximum degradation performance of OMWW was achieved by Ganoderma lucidum, achieving 19.4% phenols reduction together with 47.56% decolorization. The extracellular polysaccharides (EPS) produced by all strains were found to be richer in total glucans during growth in semi-synthetic medium, compared to growth in OMWW-based medium. In regard to biomass polysaccharides, Pleurotus ostreatus biomass was found to be richer in glucans, reaching 8.68% (w/w) total glucan content when grown in semi-synthetic medium and 7.58% (w/w) when grown in OMWW-based medium. After purification of biomass polysaccharides with two methods, the fraction with the highest glucan content was found to be the one from G. lucidum after growth in semi-synthetic medium cultures, with 49.1% (w/w) total glucans. FT-IR spectra of the isolated samples revealed the bands corresponding to α- and β-glucosidic bonds, but also the existence of protein contamination.
Conclusions
Purification of biomass polysaccharides with two distinct methods revealed that α-amylase and Sevag treatments failed to remove completely α-glucans and proteins respectively, leading to the suggestion that these two steps could be omitted without significant impact. Moreover, the results imply that the valorization of OMWW might be feasible with the use of mushroom strains, leading to the production of important products, such as glucans.



Similar content being viewed by others
References
Rop, O., Mlcek, J., Jurikova, T.: Beta-glucans in higher fungi and their health effects. Nutr. Rev. 67(11), 624–631 (2009)
Synytsya, A., Novak, M.: Structural analysis of glucans. Ann. Transl. Med. 2(2), 17 (2014)
Chen, J., Seviour, R.: Medicinal importance of fungal beta-(1–>3), (1–>6)-glucans. Mycol. Res. 111, 635–652 (2007)
Kim, Y.W., Kim, K.H., Choi, H.J., Lee, D.S.: Anti-diabetic activity of β-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol. Lett. 27(7), 483–487 (2005)
Chai, R., Qiu, C., Liu, D., Qi, Y., Gao, Y., Shen, J., Qiu, L.: β-Glucan synthase gene overexpression and β-glucans overproduction in Pleurotus ostreatus using promoter swapping. PLoS ONE. 8(4), e61693 (2013)
Carbonero, E. R., Gracher, A. H. P., Smiderle, F. R., Rosado, F. R., Sassaki, G. L., Gorin, P. A. J., Iacomini, M.: A β-glucan from the fruit bodies of edible mushrooms Pleurotus eryngii and Pleurotus ostreatoroseus. Carbohydr. Polym. 66(2), 252–257 (2006)
Gao, Y., Gao, Η., Chan, Ε., Tang, W., Xu, A., Yang, H., Huang, M., Lan, J., Li, X., Xu, C., Zhou, S., Duan, W.: Antitumor activity and underlying mechanisms of ganopoly, the refined polysaccarides extracted from Ganoderma lucidum, in mice. Immunol. Investig. 34(2), 171–198 (2005)
Santos-Neves, J. C., Pereira, M. I., Carbonero, E. R., Gracher, A. H. P., Gorin, P. A. J., Sassaki, G. L., Iacomini, M.: A gel-forming β-glucan isolated from the fruit bodies of the edible mushroom Pleurotus florida. Carbohydr. Res. 343(9), 1456–1462 (2008)
Synytsya, A., Míčková, K., Synytsya, A., Jablonský, I., Spěváček, J., Erban, V., Kováříková, E., Čopíková, J.: Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 76(4), 548–556 (2009)
Wang, J. C., Hu, S. H., Liang, Z. C., Yeh, C. J.: Optimization for the production of water-soluble polysaccharide from Pleurotus citrinopileatus in submerged culture and its antitumor effect. Appl. Microbiol. Biotechnol. 67(6), 759–766 (2005)
Reverberi, M., Di Mario, F., Tomati, U: β-Glucan synthase induction in mushrooms grown on olive mill wastewaters. Appl. Microbiol. Biotechnol. 66(2), 217–225 (2004)
Crognale, S., Federici, F., Petruccioli, M.: beta-Glucan production by Botryosphaeria rhodina on undiluted olive-mill wastewaters. Biotechnol. Lett. 25(23), 2013–2015 (2003)
Zerva, A., Zervakis, G. I., Christakopoulos, P., Topakas, E.: Degradation of olive mill wastewater by the induced extracellular ligninolytic enzymes of two wood-rot fungi. J. Environ. Man. (2016). doi:10.1016/j.jenvman.2016.02.042
Belardinelli, M., Galli, E., Tomati, U.: Lentinan production by Lentinula edodes grown in oil mill wastewaters. In: Proceedigs of World Conference on Biomass for Energy and Industry, vol. 1, p. 251 (2000)
Agalias, A., Magiatis, P., Skaltsounis, A. L., Mikros, E., Tsarbopoulos, A., Gikas, E., Spanos, I., Manios, T.: A new process for the management of olive oil mill waste water and recovery of natural antioxidants. J. Agric. Food. Chem. 55(7), 2671–2676 (2007)
Sarris, D., Matsakas, L., Aggelis, G., Koutinas, A. A., Papanikolaou, S.: Aerated vs non-aerated conversions of molasses and olive mill wastewaters blends into bioethanol by Saccharomyces cerevisiae under non-aseptic conditions. Ind. Crops Prod. 56, 83–93 (2014)
Diamantis, V., Erguder, T. H., Aivasidis, A., Verstraete, W., Voudrias, E.: Wastewater disposal to landfill-sites: A synergistic solution for centralized management of olive mill wastewater and enhanced production of landfill gas. J. Environ. Manage. 128, 427–434 (2013)
Arvanitoyannis, I.S., Kassaveti, A., Stefanatos, S.: Olive oil waste treatment: a comparative and critical presentation of methods, advantages & disadvantages. Crit. Rev. Food Sci. Nutr 47, 187–229 (2007)
D’Annibale, A., Sermanni, G.G., Federici, F., Petruccioli, M.: Olive-mill wastewaters: a promising substrate for microbial lipase production. Bioresour. Technol. 97, 1828–1833 (2006)
Ntaikou, I., Kourmentza, C., Koutrouli, E.C., Stamatelatou, K., Zampraka, A., Kornaros, M., Lyberatos, G.: Exploitation of olive oil mill wastewater for combined biohydrogen and biopolymers production. Bioresour. Technol. 100, 3724–3730 (2009)
Khoufi, S., Hamza, M., Sayadi, S.: Enzymatic hydrolysis of olive wastewater for hydroxytyrosol enrichment. Bioresour. Technol. 102, 9050–9058 (2011)
Paredes, C., Bernal, M. P., Roig, A., Cegarra, J.: Effects of olive mill wastewater addition in composting of agroindustrial and urban wastes. Biodegradation. 12(4), 225–234 (2001)
Ntougias, S., Gaitis, F., Katsaris, P., Skoulika, S., Iliopoulos, N., Zervakis, G.I.: The effects of olives harvest period and production year on olive mill wastewater properties: evaluation of Pleurotus strains as bioindicators of the effluent’s toxicity. Chemosphere. 92, 399–405 (2013). doi:10.1016/j.chemosphere.2013.01.033
Koutrotsios, G., Zervakis, G.I.: Comparative examination of the olive mill wastewater biodegradation process by various wood-rot macrofungi. BioMed Res. Int. (2014). doi:10.1155/2014/482937
Papaspyridi, L. M., Katapodis, P., Gonou-Zagou, Z., Kapsanaki-Gotsi, E., Christakopoulos, P.: Growth and biomass production with enhanced β-glucan and dietary fibre contents of Ganoderma australe ATHUM 4345 in a batch-stirred tank bioreactor. Eng. Life Sci. 11(1), 65–74 (2011)
Waterhouse, A.L., 2001. Determination of total phenolics, in: John Wiley & Sons, Inc. (Eds.), Current protocols in food analytical chemistry.
Aggelis, G., Ehaliotis, C., Nerud, F., Stoychev, I., Lyberatos, G., Zervakis, G.: Evaluation of white-rot fungi for detoxification and decolorization of effluents from the green olive debittering process. Appl. Microbiol. Biotechnol. 59, 353–360 (2002)
Wei, S., Helsper, J. P. F. G., Van Griensven, L. J. L. D.: Phenolic compounds present in medicinal mushroom extracts generate reactive oxygen species in human cells in vitro. Int. J. Med. Mushrooms. 10(1), 1–13 (2008)
Han, M. D., Han, Y. S., Hyun, S. H., Shin, H. W.: Solubilization of water-insoluble beta-glucan isolated from Ganoderma lucidum. J. Environ. Biol. 29(2), 237–242 (2008)
Fan, Y., He, X., Zhou, S., Luo, A., He, T., Chun, Z.: Composition analysis and antioxidant activity of polysaccharide from Dendrobium denneanum. Int. J. Biol. Macromol. 45(2), 169–173 (2009)
Olivieri, G., Russo, M., Giardina, P., Marzocchella, A., Sannia, G., Salatino, P.: Strategies for dephenolization of raw olive mill wastewater by means of Pleurotus ostreatus. J. Ind. Microbiol. Biotechnol. 39(5), 719–729 (2012)
Tsioulpas, A., Dimou, D., Iconomou, D., Aggelis, G.: Phenolic removal in olive oil mill wastewater by strains of Pleurotus spp. in respect to their phenol oxidase (laccase) activity. Bioresour. Technol. 84(3), 251–257 (2002)
Fan, L., Soccol, C. R., Pandey, A.: Mushroom production. In: Pandey, A., Soccol, C. R., Larroche, C.. (eds.) Current developments in solid-state fermentation, pp. 253–274. Springer, New York (2008)
Lee, B. C., Bae, J. T., Pyo, H. B., Choe, T. B., Kim, S. W., Hwang, H. J., Yun, J. W.: Biological activities of the polysaccharides produced from submerged culture of the edible basidiomycete Grifola frondosa. Enzyme Microb. Technol. 32(5), 574–581 (2003)
Liu, X., Zhou, B., Lin, R., Jia, L., Deng, P., Fan, K., Wang, G., Wang, L., Zhang, J.: Extraction and antioxidant activities of intracellular polysaccharide from Pleurotus sp. mycelium. Int. J. Biol. Macromol. 47(2), 116–119 (2010)
Smiderle, F. R., Carbonero, E. R., Mellinger, C. G., Sassaki, G. L., Gorin, P. A. J., Iacomini, M.: Structural characterization of a polysaccharide and a β-glucan isolated from the edible mushroom Flammulina velutipes. Phytochemistry. 67(19), 2189–2196 (2006)
Tong, H., Xia, F., Feng, K., Sun, G., Gao, X., Sun, L., Jiang, R., Tian, D., Sun, X.: Structural characterization and in vitro antitumor activity of a novel polysaccharide isolated from the fruiting bodies of Pleurotus ostreatus. Bioresour. Technol. 100(4), 1682–1686 (2009)
Amaral, A.E., Carbonero, E.R., Simao R.d.C.G., Kadowaki, M.K., Sassaki, G.L., Osaku, C.A., Gorin, P.A.J., Lacomini, M.: An unusual water-soluble β-glucan from the basidiocarp of the fungus Ganoderma resinaceum. Carbohydr. Polym. 72(3), 473–478 (2008)
Lee, Y.L., Huang, G.W., Liang, Z.C., Mau, J.L.: Antioxidant properties of three extracts from Pleurotus citrinopileatus. LWT-Food Sci. Technol. 40(5), 823–833 (2007)
Mu, H., Zhang, A., Zhang, W., Cui, G., Wang, S., Duan, J.: Antioxidative Properties of Crude Polysaccharides from Inonotus obliquus. Int. J. Mol. Sci. 13(7), 9194–9206 (2012)
Acknowledgements
The authors would like to thank Associate Prof. George Zervakis from the Agricultural University of Athens for kindly providing the basidiomycetes used in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zerva, A., Papaspyridi, LM., Christakopoulos, P. et al. Valorization of Olive Mill Wastewater for the Production of β-glucans from Selected Basidiomycetes. Waste Biomass Valor 8, 1721–1731 (2017). https://doi.org/10.1007/s12649-017-9839-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9839-7