Abstract
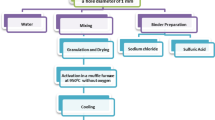
In the present study free and immobilized on magnetic nanoparticles coated with chitosan (C-MNP) laccase was applied to validate their effects on agave biomass waste as pretreatment before enzymatic saccharification. C-MNP were prepared by one step coprecipitation. Trametes versicolor laccase was immobilized via glutaraldehyde reaction. Fourier-Transformed Infrared spectra, magnetization measurements, and high resolution transmission electron microscope were performed to characterize bio-nanocomposite. Operational properties of free and immobilized laccase were evaluated spectrophotometrically using catechol as substrate. Effects of free and immobilized laccase on Agave atrovirens biomass waste, pretreated previously by autoclaving, were valued by glucose release after cellulase catalyzed hydrolysis. Immobilization technique yielded 82% of protein immobilization, and 89% of initial activity were retained. Immobilized enzyme showed better thermal and storage stability than free enzyme; Km value increased only at 1.25 times, and its activity behavior as pH and temperature function was maintained. Immobilized laccase retained 50% of its activity after 5 cycles. Free laccase applied for 12 h at 3 U (solid vegetal material g−1) in cactus Agave atrovirens biomass led to raise the yield of enzymatic hydrolysis (with Celluclast at 15 U g−1) at 11%, while immobilized laccase led to decrease yield at 16%. It was demonstrated that the interaction between bio-nanocomposite and lignocellulosic material probably hindered magnetic separation of immobilized enzyme. Thus, free laccase may be applied as agave biomass treatment before enzymatic hydrolysis. Immobilized enzyme may be applied for other biotechnological processes where magnetic separation may be useful for development of new technologies.
Graphical Abstract








Similar content being viewed by others
References
Zhang, Y., Rochefort, D.: Activity, conformation and thermal stability of laccase and glucose oxidase in poly(ethyleneimine) microcapsules for immobilization in paper. Process Biochem. 46, 993–1000 (2011)
Gutiérrez, A., Rencoret, J., Cadena, E.M., Rico, A., Barth, D., del Río, J.C., Martínez, Á.T.: Demonstration of laccase-based removal of lignin from wood and non-wood plant feedstocks. Bioresour. Technol. 119, 114–122 (2012)
Netto, C.G.C.M., Toma, H.E., Andrade, L.H.: Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. J. Mol. Catal. B Enzym. 85–86, 71–92 (2013)
Tavares, A.P.M., Rodríguez, O., Fernández-Fernández, M., Domínguez, A., Moldes, D., Sanromán, M.A., Macedo, E.A.: Immobilization of laccase on modified silica: stabilization, thermal inactivation and kinetic behaviour in 1-ethyl-3-methylimidazolium ethylsulfate ionic liquid. Bioresour. Technol. 131, 405–412 (2013)
Bourbonnais, R., Paice, M.: Demethylation and delignification of kraft pulp by Trametes versicolor laccase in the presence of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate). Appl. Microbiol. Biotechnol. 36, 823–827 (1992)
Fernández-Fernández, M., Sanromán, M.Á., Moldes, D.: Recent developments and applications of immobilized laccase. Biotechnol. Adv. 31, 1808–1825 (2013)
Rodríguez Couto, S., Herrera, J.L.T.: Industrial and biotechnological applications of laccases: a review. Biotechnol. Adv. 24, 500–513 (2006)
Rotková, J., Šuláková, R., Korecká, L., Zdražilová, P., Jandová, M., Lenfeld, J., Horák, D., Bílková, Z.: Laccase immobilized on magnetic carriers for biotechnology applications. J. Magn. Magn. Mater. 321, 1335–1340 (2009)
Demarche, P., Patel, H., Gupte, S.: Harnessing the power of enzymes for enviromental stewardship. Biotechnol. Adv. 30, 933–953 (2012)
Thakur, S., Patel, H., Gupte, S., Gupte, A.: Laccases: the biocatalyst with industrial and biotechnological applications. In: Satyanarayana, T., Johri, B.N. (eds.) Microorganisms in sustainable agriculture and biotechnology, pp. 309–342. Springer, Netherlands (2012)
Camarero, S., Ibarra, D., Martínez, Á.T., Romero, J., Gutiérrez, A., del Río, J.C.: Paper pulp delignification using laccase and natural mediators. Enzym. Microb. Technol. 40, 1264–1271 (2007)
Feng, C., Zeng, G., Huang, D., Hu, S., Zhao, M., Lai, C., Huang, C., Wei, Z., Li, N.: Effect of ligninolytic enzymes on lignin degradation and carbon utilization during lignocellulosic waste composting. Process Biochem. 46, 1515–1520 (2011)
Moilanen, U., Kellock, M., Galkin, S., Viikari, L.: The laccase-catalyzed modification of lignin for enzymatic hydrolysis. Enzym. Microb. Technol. 49, 492–498 (2011)
Yu, Z., Jameel, H., Chang, H.M., Park, S.: The effect of delignification of forest biomass on enzymatic hydrolysis. Bioresour. Technol. 102, 9083–9089 (2011)
Sun, Y., Cheng, J.: Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour. Technol. 83, 1–11 (2002)
Barr, D.P., Aust, S.D.: Mechanisms white rot fungi use to degrade pollutants. Environ. Sci. Technol. 28, 78A–87A (1994)
Datta, S., Christena, L.R., Rajaram, Y.: Enzyme immobilization: an overview on techniques and support materials. 3 Biotech 3, 1–9 (2013)
de Moraes Rocha, G.J., Nascimento, V.M., da Silva, V.F.N.: Enzymatic bioremediation of effluent from sugarcane bagasse soda delignification process. Waste Biomass Valoriz. 5, 919–929 (2014)
Lu, A.H., Salabas, E.L., Schüth, F.: Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 46, 1222–1244 (2007)
Sánchez-Ramírez, J., Iliná, A., Segura-Ceniceros, E.P., Aguilar, C.N., Medina-Morales, M.A., Martínez-Hernández, J.L.: Influencia de pretratamientos en la bioconversión enzimática de fibras de pencas de Agave. Rev. Facul. Nac. Agron. Medellín. 67, 915–916 (2014)
Zhao, F., Zhang, B., Feng, L.: Preparation and magnetic properties of magnetite nanoparticles. Mater. Lett. 68, 112–114 (2012)
Bayramoglu, G., Yilmaz, M., Yakup Arica, M.: Preparation and characterization of epoxy-functionalized magnetic chitosan beads: laccase immobilized for degradation of reactive dyes. Bioprocess Biosyst. Eng. 33, 439–448 (2010)
Fang, H., Huang, J., Ding, L., Li, M., Chen, Z.: Preparation of magnetic chitosan nanoparticles and immobilization of laccase. J. Wuhan Univ. Technol. Mater. Sci. Ed. 24, 42–47 (2009)
Huan, W., Yang, Y., Wu, B., Yuan, H., Zhang, Y., Liu, X.: Degradation of 2,4-DCP by the Immobilized laccase on the carrier of Fe3O4@SiO2-NH2. Chin. J. Chem. 30, 2849–2860 (2012)
Jiang, D.S., Long, S.Y., Huang, J., Xiao, H.Y., Zhou, J.Y.: Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem. Eng. J. 25, 15–23 (2005)
Kalkan, N.A., Aksoy, S., Aksoy, E.A., Hasirci, N.: Preparation of chitosan-coated magnetite nanoparticles and application for immobilization of laccase. J. Appl. Polym. Sci. 123, 707–716 (2012)
Wang, F., Guo, C., Yang, L.R., Liu, C.Z.: Magnetic mesoporous silica nanoparticles: fabrication and their laccase immobilization performance. Bioresour. Technol. 101, 8931–8935 (2010)
Osuna, Y., Gregorio-Jauregui, K.M., Gaona, L.G., De la Garza, R.I.M., Ilina, A., Barriga, C.E.D., Saade, H., Lopez, R.G.: Chitosan-coated magnetic nanoparticles with low chitosan content prepared in one-step. J. Nanomater. (2012). doi:10.1155/2012/327562
Bradford, M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976)
Kaiser, E., Colescott, R.L., Bossinger, C.D., Cook, P.I.: Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 34, 595–598 (1970)
Hu, X., Zhao, X., Hwang, H.M.: Comparative study of immobilized Trametes versicolor laccase on nanoparticles and kaolinite. Chemosphere 66, 1618–1626 (2007)
Wang, F., Guo, C., Liu, H.Z., Liu, C.Z.: Immobilization of Pycnoporus sanguineus laccase by metal affinity adsorption on magnetic chelator particles. J. Chem. Technol. Biotechnol. 83, 97–104 (2008)
Sánchez-Ramírez, J., Martínez-Hernández, J.L., Segura-Ceniceros, E.P., Contreras-Esquivel, J.C., Medina-Morales, M.A., Aguilar, C.N., Iliná, A.: Inmovilización de enzimas lignocelulolíticas en nanopartículas magnéticas. Quim. Nova 37, 504–512 (2014)
Li, A., Antizar-Ladislao, B., Khraisheh, M.: Bioconversion of municipal solid waste to glucose for bio-ethanol production. Bioprocess Biosyst. Eng. 30, 189–196 (2007)
Wu, Y., Wang, Y., Luo, G., Dai, Y.: In situ preparation of magnetic Fe3O4-chitosan nanoparticles for lipase immobilization by cross-linking and oxidation in aqueous solution. Bioresour. Technol. 100, 3459–3464 (2009)
Gregorio-Jauregui, K., Carrizalez-Alvarez, S., Rivera-Salinas, J., Saade, H., Martinez, J., López, R., Segura, E., Ilyina, A.: Extraction and immobilization of SA-α-2,6-gal receptors on magnetic nanoparticles to study receptor stability and interaction with Sambucus nigra lectin. Appl. Biochem. Biotechnol. 172, 3721–3735 (2014)
Gregorio-Jauregui, K.M., Pineda, M.G., Rivera-Salinas, J.E., Hurtado, G., Saade, H., Martinez, J.L., Ilyina, A., López, R.G.: One-step method for preparation of magnetic nanoparticles coated with chitosan. J. Nanomater. (2012). doi:10.1155/2012/813958
Sánchez-Ramírez, J., Martínez-Hernández, J.L., Segura-Ceniceros, P., López, G., Saade, H., Medina-Morales, M.A., Ramos-González, R., Aguilar, C.N., Ilyina, A.: Cellulases immobilization on chitosan-coated magnetic nanoparticles: application for Agave Atrovirens lignocellulosic biomass hydrolysis. Bioprocess Biosyst. Eng. (2016). doi:10.1007/s00449-016-1670-1
Arroyo, M.: Inmovilización de enzimas. Fundamentos, métodos y aplicaciones. Ars Pharm. 39, 23–39 (1998)
Jordan, J., Kumar, C.S.S.R., Theegala, C.: Preparation and characterization of cellulase-bound magnetite nanoparticles. J. Mol. Catal. B Enzym. 68, 139–146 (2011)
Liu, Y., Zeng, Z., Zeng, G., Tang, L., Pang, Y., Li, Z., Liu, C., Lei, X., Wu, M., Ren, P., Liu, Z., Chen, M., Xie, G.: Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of phenolic compounds. Bioresour. Technol. 115, 21–26 (2012)
Ben Hamissa, A.M., Seffen, M., Aliakbarian, B., Casazza, A.A., Perego, P., Converti, A.: Phenolics extraction from Agave americana (L.) leaves using high-temperature, high-pressure reactor. Food Bioprod. Process. 90, 17–21 (2012)
Medina Morales, M.A.: Hidrólisis enzimática de fibras de hoja de Agave. Ph.D. Thesis, Universidad Autónoma de Coahuila (2012)
Nava-Cruz, N., Medina-Morales, M.A., Martínez-Hernández, J.L., Rodríguez-Herrera, R., Aguilar, C.N.: Agave biotechnology: an overview. Crit. Rev. Biotechnol. 35, 546–559 (2015)
Rico, A., Rencoret, J., del Río, J.C., Martínez, A.T., Gutiérrez, A.: Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnol. Biofuels 7, 6–20 (2014)
Cañas, A.I., Camarero, S.: Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 28, 694–705 (2010)
Palonen, H., Viikari, L.: Role of oxidative enzymatic treatments on enzymatic hydrolysis of softwood. Biotechnol. Bioeng. 86, 550–557 (2004)
Jørgensen, H., Kristensen, J.B., Felby, C.: Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels, Bioprod. Biorefin. 1, 119–134 (2007)
Alvira, P., Tomás-Pejó, E., Ballesteros, M., Negro, M.J.: Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol. 10, 4851–4861 (2010)
Acknowledgements
The authors would thank the Mexican Council of Science and Technology (CONACYT) for its financial support to carry out this investigation project, Grant No. 213844 (PDCPN2013-01 CONACYT-Mexico), as well as for the financial support under the program “Cátedras CONACYT - 2015” (Project No. 729).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez-Ramírez, J., Martínez-Hernández, J.L., López-Campos, R.G. et al. Laccase Validation as Pretreatment of Agave Waste Prior to Saccharification: Free and Immobilized in Superparamagnetic Nanoparticles Enzyme Preparations. Waste Biomass Valor 9, 223–234 (2018). https://doi.org/10.1007/s12649-016-9774-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9774-z