Abstract
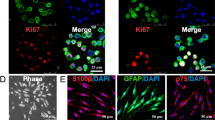
Nowadays, researchers pay a vast deal of attention to neural tissue regeneration due to its tremendous effect on the patient’s life. There are many strategies, from using conventional autologous nerve grafts to the newly developed methods for reconstructing damaged nerves. Among the various therapeutic methods, incorporating highly potent biomolecules and growth factors, the damaged nerve site would promote nerve regeneration. The aim was to examine the efficiency of a mesenchymal stem cell condition medium (MSC-CM) loaded on a 3D-polycaprolactone (PCL) scaffold as a nerve conduit in an axotomy rat model. Twenty-four mature male rats were classified into four groups: controls (the animals of this group were intact), axotomy (10 mm piece of the nerve was removed), axotomy (10-mm piece of the nerve was removed) + scaffold, and axotomy (10-mm piece of the nerve was removed) + MSC-CM-loaded scaffold. We followed up nerve motor function using a sciatic function index and electromyography activity of the gastrocnemius muscle. At 12 weeks post axotomy, sciatic nerve and dorsal root ganglion specimens and L4 and L5 spinal cord segments were separated from the rats and were analyzed by stereological, immunohistochemistry, and RT-PCR procedures. The rats of the axotomy group presented the expected gross locomotor deficit. Stereological parameters, immunohistochemistry of GFAP, and gene expression of S100, NGF, and BDNF were significantly enhanced in the CM-loaded scaffold group compared with the axotomy group. The most observed similarity was noted between the results of the control group and the CM-loaded scaffold group. Our results support the potential applicability of MSC-CM-loaded PCL nanofibrous scaffold to treat peripheral nerve injury (PNI).











Similar content being viewed by others
References
Ahmadi H, Boroujeni ME, Sadeghi Y, Abdollahifar MA, Khodagholi F, Meftahi GH, Hadipour M, Bayat AH, Shaerzadeh F, Aliaghaei A (2018) Sertoli cells avert neuroinflammation-induced cell death and improve motor function and striatal atrophy in rat model of Huntington disease. J Mol Neurosci 65:17–27
Bain J, Mackinnon S, Hunter D (1989) Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83:129–138
Barde YA (1989) Trophic factors and neuronal survival. Neuron 2:1525–1534
Bierlein Del, Rosa M, Sharma AD, Mallapragada SK, Sakaguchi, DS (2017) Transdifferentiation of brain-derived neurotrophic factor (BDNF)-secreting mesenchymal stem cells significantly enhance BDNF secretion and Schwann cell marker proteins. J Biosci Bioeng 124:572–582
Bischoff B, Romstock J, Fahlbusch R, Buchfelder M, Strauss C (2008) Intraoperative brainstem auditory evoked potential pattern and perioperative vasoactive treatment for hearing preservation in vestibular schwannoma surgery. J Neurol Neurosurg Psychiatry 79:170–175
Boyd JG, Gordon T (2003) Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol 183:610–619
Chernov AV, Dolkas J, Hoang K, Angert M, Srikrishna G, Vogl T, Baranovskaya S, Strongin AY, Shubayev VI (2015) The calcium-binding proteins S100A8 and S100A9 initiate the early inflammatory program in injured peripheral nerves. J Biol Chem 290:11771–11784
Chong EJ, Phan TT, Lim IJ, Zhang Y, Bay BH, Ramakrishna S, Lim CT (2007) Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomaterialia 3:321–330
Ding F, Wu J, Yang Y, Hu W, Zhu Q, Tang X, Liu J, Gu X (2010) Use of tissue-engineered nerve grafts consisting of a chitosan/poly(lactic-co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng Part A 16:3779–3790
Ganji R, Piryaei A, Bayat M, Rajabibazl M, Mohsenifar Z, Kheirjoo R (2014) The effect of human bone marrow-mesenchymal stem cells secretoms on diabetic wound healing. Res Med 38:10–18
Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani M-H, Ramakrishna S (2008) Electrospun poly (ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 29:4532–4539
Griffin MD, Ritter T, Mahon BP (2010) Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther 21:1641–1655
Grinsell D, Keating C (2014) Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Res Int
Himes B, Tessler A (1989) Death of some dorsal root ganglion neurons and plasticity of others following sciatic nerve section in adult and neonatal rats. J Comp Neurol 284:215–230
Horie H, Kadoya T, Hikawa N, Sango K, Inoue H, Takeshita K, Asawa R, Hiroi T, Sato M, Yoshioka T (2004) Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J Neurosci 24:1873–1880
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736
IDE C (1996) Peripheral nerve regeneration. Neurosci Res 25:101–121
Johnson EO, Charchanti A, Soucacos PN (2008) Nerve repair: experimental and clinical evaluation of neurotrophic factors in peripheral nerve regeneration. Injury 39(Suppl 3):S37-42
Karaoz E, Aksoy A, Ayhan S, Sarıboyaı AE, Kaymaz F, Kasap M (2009) Characterization of mesenchymal stem cells from rat bone marrow: ultrastructural properties, differentiation potential and immunophenotypic markers. Histochem Cell Biol 132:533
Kim CH, Khil MS, Kim HY, Lee HU, Jahng KY (2006a) An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J Biomed Mater Res Part b: Appl Biomater: an Official Journal of the Society for Biomaterials, the Japanese Society for Biomaterials, and the Australian Society for Biomaterials and the Korean Society for Biomaterials 78:283–290
Kim CH, Khil MS, Kim HY, Lee HU, Jahng KY (2006b) An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J Biomed Mater Res B Appl Biomater 78:283–290
Koh H, Yong T, Chan C, Ramakrishna S (2008) Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials 29:3574–3582
Ladak A, Olson J, Tredget E, Gordon T (2011) Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol 228:242–252
Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, Shin JW (2005) Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials 1261–1270:26
Li WJ, Cooper Jr JA, Mauck RL, Tuan RS (2006) Fabrication and characterization of six electrospun poly (α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomaterialia 2:377–385
Lin G, Zhang H, Sun F, Lu Z, Reed-Maldonado A, Lee YC, Wang G, Banie L, Lue TF (2016) Brain-derived neurotrophic factor promotes nerve regeneration by activating the JAK/STAT pathway in Schwann cells. Transl Androl Urol 5:167–175
Lindsay RM (1988) Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci 8:2394–2405
Ma F, Xiao Z, Meng D, Hou X, Zhu J, Dai J, Xu R (2014) Use of natural neural scaffolds consisting of engineered vascular endothelial growth factor immobilized on ordered collagen fibers filled in a collagen tube for peripheral nerve regeneration in rats. Int J Mol Sci 15:18593–18609
Ma PX (2004) Scaffolds for tissue fabrication. Materials Today 7:30–40
Marinescu SA, Zărnescu O, Mihai IR, Giuglea C, Sinescu RD (2014) An animal model of peripheral nerve regeneration after the application of a collagen-polyvinyl alcohol scaffold and mesenchymal stem cells. Rom J Morphol Embryol 55:891–903
Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H (1992) Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol 119:45–54
Mohammadi R, Ahsan S, Masoumi M, Amini K (2013) Vascular endothelial growth factor promotes peripheral nerve regeneration after sciatic nerve transection in rat. Chin J Traumatol 16:323–329
Morales MM (2007) Terapias avançadas: células-tronco, terapia gênica e nanotecnologia aplicada à saúde. Sang WH. Introdução. História da terapia gênica, estado da arte, técnicas e ética. São Paulo: Atheneu, 105–25
Noorafshan A, Abdollahifar MA, Asadi-Golshan R, Rashidian-Rashidabadi A, Karbalay-Doust S (2014) Curcumin and sertraline prevent the reduction of the number of neurons and glial cells and the volume of rats’ medial prefrontal cortex induced by stress. Acta Neurobiol Exp (wars) 74:44–53
Patro N, Shrivastava M, Tripathi S, Patro IK (2009) S100β upregulation: A possible mechanism of deltamethrin toxicity and motor coordination deficits. Neurotoxicol Teratol 31:169–176
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. NAR 30:e36
Pierucci A, de Oliveira ALR (2006) Increased sensory neuron apoptotic death 2 weeks after peripheral axotomy in C57BL/6J mice compared to A/J mice. Neurosci Lett 396:127–131
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Pourmohammadi N, Alimoradi H, Mehr SE, Hassanzadeh G, Hadian MR, Sharifzadeh M, Bakhtiarian A, Dehpour AR (2012) Lithium attenuates peripheral neuropathy induced by paclitaxel in rats. Basic Clin Pharmacol Toxicol 110:231–237
Prabhakaran MP, Venugopal JR, Ramakrishna S (2009) Mesenchymal stem cell differentiation to neuronal cells on electrospun nanofibrous substrates for nerve tissue engineering. Biomaterials 30:4996–5003
Pu LL, Syed SA, Reid M, Patwa H, Goldstein JM, Forman DL, Thomson JG (1999) Effects of nerve growth factor on nerve regeneration through a vein graft across a gap. Plast Reconstr Surg 104:1379–1385
Putcha GV, Johnson EM (2004) ‘Men are but worms:’ neuronal cell death in C. elegans and vertebrates. Cell Death Differ 11:38–48
Rodríguez FJ, Valero-Cabre A, Navarro X (2004) Regeneration and functional recovery following peripheral nerve injury. Drug Discov Today: Dis Models 1:177–185
Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, Mey J (2007) Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials 28:3012–3025
Shih YR, Chen CN, Tsai SW, Wang YJ, Lee OK (2006) Growth of mesenchymal stem cells on electrospun type I collagen nanofibers. Stem Cells 24:2391–2397
Siemionow M, Brzezicki G (2009) Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol 87:141–172
Simões MJ, Amado S, Gärtner A, Armada-Da-Silva PA, Raimondo S, Vieira M, Luís AL, Shirosaki Y, Veloso AP, Santos JD (2010) Use of chitosan scaffolds for repairing rat sciatic nerve defects. Ital J Anat Embryol 115:190
Simões MJ, Amado S, Raimondo S, Armada-Da-Silva PAS, Gärtner A (2010b) Use of chitosan scaff olds for repairing rat sciatic nerve defects. http://digital.casalini.it/2490996
Sladjana UZ, Ivan JD, Bratislav SD (2008) Microanatomical structure of the human sciatic nerve. Surg Radiol Anat 30:619–626
Takemura Y, Imai S, Kojima H, Katagi M, Yamakawa I, Kasahara T, Urabe H, Terashima T, Yasuda H, Chan L, Kimura H, Matsusue Y (2012) Brain-derived neurotrophic factor from bone marrow-derived cells promotes post-injury repair of peripheral nerve. PLoS One 7, e44592
Tao SC, Guo SC, Collett J (2019) A novel role for extracellular vesicles in cytopathology and new therapeutic strategies. Biomed Res Int 2019:7137613
Terenghi G (1999) Peripheral nerve regeneration and neurotrophic factors. J Anat 194:1–14
Vargel I (2009) Impact of vascularization type on peripheral nerve microstructure. J Reconstr Microsurg 25:243–253
Vestergaard S, Tandrup T, Jakobsen J (1997) Effect of permanent axotomy on number and volume of dorsal root ganglion cell bodies. J Comp Neurol 388:307–312
Wright KT, Masri WE, Osman A, Chowdhury J, Johnson WE (2011) Concise review: bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells 29:169–178
Xin X, Hussain M, Mao JJ (2007) Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials 28:316–325
Yang L, Fitie CF, van der Werf KO, Bennink ML, Dijkstra PJ, Feijen J (2008) Mechanical properties of single electrospun collagen type I fibers. Biomaterials 962–955:29
Zalewski AA, Gulati AK (1981) Rejection of nerve allografts after cessation of immunosuppression with cyclosporin A. Transplantation 31:88
Acknowledgements
This work was performed at the School of Medicine, Shahid Beheshti University of Medical Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of Ethics
The authors declare that all experimental protocols were approved by the Ethics Committee, deputy of research, Shahid Beheshti University of Medical Sciences, Tehran, Iran. All methods were carried out following relevant guidelines and regulations.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raoofi, A., Sadeghi, Y., Piryaei, A. et al. Bone Marrow Mesenchymal Stem Cell Condition Medium Loaded on PCL Nanofibrous Scaffold Promoted Nerve Regeneration After Sciatic Nerve Transection in Male Rats. Neurotox Res 39, 1470–1486 (2021). https://doi.org/10.1007/s12640-021-00391-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00391-5