Abstract
In human addicts and in animal models, chronic cocaine use leads to numerous alterations in glutamatergic transmission, including its receptors. The present study focused on metabotropic glutamatergic receptors type 5 (mGluR5) and N-methyl-D-aspartate receptor subunits (NMDAR: GluN1, GluN2A, GluN2B) proteins during cocaine self-administration and after 10-day of extinction training in rats. To discriminate the contingent from the non-contingent cocaine delivery, we employed the “yoked”-triad control procedure. Protein expression in rat prefrontal cortex, nucleus accumbens, hippocampus, and dorsal striatum was determined. We also examined the Homer1b/c protein, a member of the postsynaptic density protein family that links NMDAR to mGluR5. Our results revealed that cocaine self-administration selectively increased GluN1 and GluN2A subunit in the rat hippocampus and dorsal striatum, respectively, while mGluR5 protein expression was similarly increased in the dorsal striatum of both experimental groups. Withdrawal from both contingent and non-contingent cocaine delivery induced parallel increases in prefrontal cortical GluN2A protein expression, hippocampal mGluR5, and GluN1 protein expression as well as in accumbal GluN1 subunit expression, while the mGluR5 expression was reduced in the prefrontal cortex. Extinction training in animals with a history of cocaine self-administration resulted in an elevation of the hippocampal GluN2A/GluN2B subunits and accumbal mGluR5, and in a 50 % decrease of mGluR5 protein expression in the dorsal striatum. The latter reduction was associated with Homer1b/1c protein level decrease. Our results showed that both contingent and non-contingent cocaine administration produces numerous, brain region specific, alterations in the mGluR5, NMDA, and Homer1b/1c protein expression which are dependent on the modality of cocaine administration.
Similar content being viewed by others
Introduction
Cocaine belongs to the most commonly used illicit stimulant drug in Europe with about 2.2 million young drug adults aged 15–34 in 2012 (EMCDDA, 2014). At the global level, the number of adult cocaine users (age 15–64) ranges from 13.3 to 19.7 million (UNDCP, 2012): this renders cocaine use disorder a significant health problem resulting in a large number of medical, psychological, and social problems (Kim and Lawrence 2014).
Cocaine addiction is associated with enduring neurochemical differences in the brain, including glutamate (Glu) neurotransmission (for review see: Pomierny-Chamiolo et al. 2014). In fact, in preclinical models, the expression of drug seeking, modeling the core feature of cocaine addiction, promotes Glu release in the ventral tegmental area and the core of the nucleus accumbens (Madhavan et al. 2013). The release of Glu during cocaine seeking also elicits rapid postsynaptic changes in proteins regulating Glu signaling and surface spine morphology, while attenuation of Glu transmission reduces drug reinforcement and relapse-like behavior (McFarland et al. 2003; Brebner et al. 2005; Gipson et al. 2013). The potentiation of Glu transmission, from prefrontal glutamatergic neurons to the accumbal core during drug-seeking behaviors, is also critical to drug-associated memories (Kalivas 2009). On the other hand, withdrawal from cocaine treatment alters the number of accumbal Glu synapses and spine density associated with a deteriorating actin cytoskeleton and a reduction in Glu signaling-related proteins (Shen et al. 2009). As shown in rodents, these changes are the consequence of reduced basal accumbal extracellular Glu, together with changes in mechanisms responsible for Glu clearance (Pomierny-Chamiolo et al. 2014).
Cocaine-induced fluctuations in glutamatergic transmission contribute to significant adaptations in glutamatergic receptors, including ionotropic N-methyl-D-aspartate receptors (NMDAR) and metabotropic glutamatergic receptors type 5 (mGluR5) in cortical and subcortical brain areas (Volkow et al. 2003; Tzschentke and Schmidt 2003) NMDARs are ionotropic channels formed by the combination of different subunits. The obligatory GluN1 is a channel-forming subunit and a site which binds co-agonists glycine or d-serine, GluN2A-D subunits have an agonist site for glutamate and other receptor agonists and antagonists, while GluN3A and B subunits may reduce Ca2+ permeability and Mg2+ sensitivity (Low and Wee 2010).
Both mGluR5 and NMDA receptors seem to be the key players in drug addiction. At the behavioral level, mGluR5 knockout mice do not acquire cocaine self-administration response, while blockade of the mGluR5 reduces cocaine self-administration in rats, cocaine-induced lethality as well as expression of behavioral sensitization in mice (Keck et al. 2014; Pomierny-Chamiolo et al. 2014). Cornish et al. (1999) reported that stimulation of NMDARs in the nucleus accumbens augments the reinforcing effect of cocaine, while inhibition of GluN2B by ifenprodil-blocked cocaine sensitization effect (Schumann and Yaka 2009).
NMDAR and mGluR5 enter into mutual interactions (Fig. 1). Connection between these receptors regulates the plasma membrane trafficking of them via the complex Shank-GKAP-PSD95 connected to the NMDAR (Scheggi et al. 2002; Ferraguti et al. 2008; Szumlinski et al. 2008; Ghasemzadeh et al. 2009a). At the functional level, it was reported that mGluR5 desensitization is mediated by activation of NMDAR (Alagarsamy et al. 2005). mGluR5-Homer-NMDA complex is importantly involved in synaptic plasticity such as LTP and LTD in drug addiction (Anwyl 1999; Ferraguti and Shigemoto 2006; Ronesi and Huber 2008; Kasanetz et al. 2010; Brown et al. 2011; Huang et al. 2011; Shen and Kalivas 2013).
Interactions between mGluR1/5 and NMDA receptors and their signaling pathways in the postsynaptic neuron. NMDA receptors are linked to mGluR1/5 receptors through complex composed of cytoplasmatic proteins PSD-95, guanylate kinase-associated protein (GKAP), Shank protein and Homer1b/c. Stimulation of mGluR1/5 receptors activates phospholipase C (PLC) and thereby leads to enhanced production of inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG) forms phosphatidylinositol-4,5-biphosphate (PIP2) and potentiates L-type voltage-dependent calcium channels (VGCC). Elevated IP3 level mobilizes Ca2+ release from internal stores. DAG contributes to activation protein kinase C (PKC) and subsequently extracellular signal-regulated protein kinases ERK1/2 phosphorylation. mGluR1/5 receptors are also coupled by means of Homer1b/c and phosphoinositide-3-kinase enhancer (PIKE-L) to phosphoinositide-3-kinase (PI3-K). All interactions involving long isoforms of Homer are disrupted by the short isoform Homer1a and thereby affect mGluR1/5 signaling. Summarizing activation of mGluR5 and NMDA receptors leads to phosphorylation transcription factors, therein cAMP response element-binding protein (CREB) and changes in gene expression
Based on these lines of evidence, we decided to investigate more in details the expression of these receptors using a different approach. The unique aspect of the current study was the investigation of the effects of contingent and non-contingent cocaine delivery as well as postcocaine drug free period with extinction training sessions (either 1 or 10 days) on the mGluR5-Homer-NMDAR in order to discriminate between the motivational and the pure pharmacological properties of cocaine. To extend few preclinical studies on the NMDAR and mGluR5 distribution after cocaine administration (Ghasemzadeh et al. 1999; Crespo et al. 2002; Hemby et al. 2005; Ary and Szumlinski 2007; Ghasemzadeh et al. 2011), we studied several rat brain areas related to reward processing (the nucleus accumbens), habit forming learning (the dorsal striatum), executive control (the prefrontal cortex), as well as learning and memory (the hippocampus) (Kalivas 2004).
Methods
Animals
Naive, male Wistar rats (280–310 g; Charles River, Germany) were housed individually in standard plastic rodent cages in a room maintained at 20 ± 1 °C and 40–50 % humidity under a 12-hour light–dark cycle (lights on at 6.00 a.m.) with free access to water and standard animal food during habituation period. Following habituation, rats were maintained on limited access to water during the initial (2 days) lever press training sessions. All procedures were conducted during the light phase of the light–dark cycle (between 8.00 a.m. and 3.00 p.m.). All the experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and with approval of the Animal Care and Use Committee at the Institute of Pharmacology, Polish Academy of Sciences in Krakow.
Drugs
Cocaine HCl (Toronto Research, Canada) was dissolved in sterile 0.9 % NaCl and given intravenously (0.1 ml/infusion).
Behavioral Experiments
Initial Training Sessions
Rats were trained to press the lever of standard operant conditioning chambers (Med-Associates, USA) under a fixed ratio (FR) 5 schedule of water reinforcement, as described previously (Pomierny-Chamiolo et al. 2013).
Surgery
Two days following lever press training and free access to water, the rats were anesthetized with ketamine HCl (75 mg/kg, Bioketan; Biowet, Puławy, Poland) and xylazine (5 mg/kg, Sedazin; Biowet, Puławy, Poland) and chronically implanted with catheters in the external jugular vein. The catheters were flushed every day with 0.1 ml of saline solution containing heparin (70 U/ml, Biochemie GmbH, Kundl, Austria) and 0.1 ml of solution of cefazolin (10 mg/ml; Biochemie BmbH, Austria). Catheter potency was tested periodically, or whenever an animal displayed behavioral outside baseline parameters.
Cocaine Self-administration Procedure
The procedure was carried out as described previously (Bystrowska et al. 2014). Cocaine (0.5 mg/kg/infusion) self-administration was conducted under FR5 schedule of reinstatement for 16-daily 2-h sessions. Animals were divided into two subgroups (see experimental design in Fig. 2). One of them (n = 8–10 rats/group) was sacrificed immediately after last cocaine self-administration session, while other group (n = 8–10 rats/group) was underwent extinction training.
Extinction Procedure
During extinction, saline was delivered and there was no presentation of the conditioned stimulus during 2-h daily sessions. On the 10th day of extinction, animals that met the extinction criterion (i.e., responses on the active lever fell to <10 % of the responses at the active lever reached during maintenance) were sacrificed immediately following the session.
“Yoked” Procedure
In the experiment, we used the “yoked” procedure, to distinguish between the pharmacological and motivational effects of cocaine intake. In this procedure, each rat actively self-administering cocaine had assigned other two rats passively receiving either cocaine or saline.
Dissection
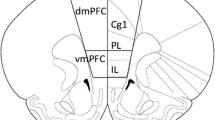
After decapitation, the brain was quickly removed and chilled in ice-cold saline. The prefrontal cortex, hippocampus, dorsal striatum, and nucleus accumbens from each animal were dissected out (Paxinos and Watson 1998) (Fig. 3). Samples were immediately frozen on dry ice, and stored at −80 °C.
Western Blot Analysis
Dissected brain structures were homogenized in 2 % sodium dodecyl sulfate (SDS) with protease and phosphatase inhibitor cocktails, 20 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM Na3VO4 (Sigma Aldrich, USA) using Ultra-Turrax homogenizer (10 s at 10,000 rpm), sonicated and then denatured for 10 min at 95 °C. After, insoluble material was removed by centrifugation at 10,000 rpm under 4 °C for 10 min. For protein determination, a bicinchoninic acid assay (BCA) protein assay kit (Thermo Scientific, Rockford, IL, USA) was used. Protein samples (35 µg) were resolved by 8 % SDS polyacrylamide gels and transferred to a polyvinylidene difluoride (PVDF) membrane. Membranes were blocked in 5 % non-fat dry milk, and separate sets of membranes were probed with rabbit anti-mGluR5 polyclonal antibody (1:2000) or mouse anti-Homer1b/c monoclonal antibody (1:500). Blots were washed and incubated in horseradish peroxidase (HRP)-conjugated secondary antibody (1:7500). Bands were detected with the ECL method using Western Bright Quantum chemiluminescent substrate (Advansta Inc., USA) and were imaged in G:Box (Syngene, USA). Next membranes were stripped using Restore Western Blot Stripping Buffer (Thermo Scientific, USA), blocked in 5 % non-fat dry milk in Tris-Buffered Saline and Tween 20 mixture (TBST) and re-probed with mouse monoclonal anti-β-actin (1:1000) or goat polyclonal anti-NR1 (1:500) using the same protocol as before. After washes, the blots were incubated in goat anti-mouse secondary antibody (1:5000) or donkey anti-goat (1:5000). After visualization of these proteins, sets of membranes were used to probe for GluN2A (mouse monoclonal; 1:500) and after then GluN2B (rabbit polyclonal; 1:400). The data were analyzed with Gene Tools (version 4.03 (a); Syngene, USA). The expressions of mGluR5, GluN1, GluN2A, GluN2B, or Homer1b/c were evaluated relative to that of ß-actin. All antibodies were obtained from Santa Cruz Biotechnology (USA), except anti-mGluR5 delivered from Millipore Bioscience Research Reagents (USA).
Data Analysis
In the behavioral procedures, the data were analyzed by Student t test (number of active and passive lever presses, infusions). In the biochemical assays, one-way ANOVA, followed by posthoc Newman–Keuls’ test, was applied to evaluate statistically significant differences between the treatment groups. To separate the effects of treatment (saline vs. cocaine) and the way of drug intake (self vs. yoked), we also used the Student t test. The criterion for statistically significant differences was set at p < 0.05.
Results
Behavioral Studies
After 16 sessions of self-administration, the rats showed stable responding on levers during the last 6 self-administration maintenance sessions with less than a 10 % difference in their daily intake of cocaine. Rats responded significantly more frequently on the active lever than on the inactive lever from the 2nd to 16th experimental session (p < 0.0001) (Fig. 4a, b). The rats self-administered either 24–37 injections of cocaine (daily mean cocaine intake between 12 and 18.5 mg/kg; group 1) or 21–39 injections (daily mean cocaine intake between 10.5–19.5 mg/kg; group 2). During 16 experimental cocaine self-administered sessions, animals received approximately 272 or 281 mg/kg/rat.
The number of active and inactive lever presses in rats that acquired and maintained cocaine (0.5 mg/kg/infusion) self-administration (left panels: a, c, e) and animals that underwent 10-day extinction training (right panels: b, d, f) with their yoked controls that received passive infusions of cocaine or saline. Data are presented as the mean ± SEM from 8 rats/group, ***p < 0.0001 versus inactive lever
During the extinction phase, neither the drug nor the drug-paired stimuli was given in response to lever pressing, which resulted in decrease in active lever presses (Fig. 4b). From day 19 to day 26 of experiment, the difference between responding on the active versus the inactive lever failed to reach significance (Fig. 4b).
In the yoked cocaine group (Fig. 4c, d), the total number of active lever presses did not differ from inactive lever. These animals passively received exactly the same amount of cocaine at the same time as the rats that learned to actively self-inject cocaine.
In the yoked saline group (Fig. 4e, f), the difference between responding on the active versus the inactive lever failed to reach significance.
Biochemical Studies
The data of protein expression for mGluR5, Homer1b/c, and NMDA receptor subunits in rat brain structures in maintenance phase and after extinction training are presented in Figs. 5, 6, 7, 8.
Effects of cocaine self-administration (a) and extinction training (b) on the mGluR5, Homer1b/1c, and NMDA (subunits: NR1, NR2A, NR2B) receptor protein expression in the rat prefrontal cortex with a “yoked” control procedure. Data were normalized to saline-treated animals (% of yoked saline; YS) and represent the mean (±SEM) of 8–10 animals/group. *p < 0.05, **p < 0.01, ***p < 0.001 versus YS. On the right, representative immunoblots for the protein levels of mGluR5, Homer1b/1c, NR1, NR2A, and NR2B are shown
mGluR5 Expression
In rats trained to self-administer cocaine (0.5 mg/kg/infusion) and those given yoked cocaine injections, a one-way ANOVA analysis showed a significant effect for mGluR5 protein expression (F(2,23) = 4.811, p = 0.0314) in the dorsal striatum. The posthoc test revealed that mGluR5 protein expression was significantly higher in both cocaine groups compared to yoked saline group (p < 0.05). (Figure 8a). A 20 % non-significant trend to increase mGluR5 protein level was also observed in the prefrontal cortex (Fig. 5a).
In animals that underwent forced abstinence from cocaine active and yoked administration, there was a significant effect in mGluR5 protein expression in the prefrontal cortex as shown by a one-way ANOVA (F(2,23) = 6.973, p = 0.048). Posthoc analyses revealed a significant decrease (ca. 30–35 %) in rats previously self-administered cocaine (p < 0.05) and those treated passively with the drug (p < 0.01) in comparison to yoked saline group (Fig. 5b). In the same animals, an opposite effect was noted in the hippocampus, as evidenced by a one-way ANOVA analysis (F(2,23) = 10.05, p = 0.0009) and by Newman–Keuls’ posthoc data (+55–85 %) in active cocaine group (p < 0.001) and yoked cocaine group (p < 0.01) versus yoked saline group (Fig. 6b).
Effects of cocaine self-administration (a) and extinction training (b) on the mGluR5, Homer1b/1c, and NMDA (subunits: NR1, NR2A, NR2B) receptor protein expression in the rat hippocampus with a “yoked” control procedure. Data were normalized to saline-treated animals (% of yoked saline; YS) and represent the mean (±SEM) of 8–10 animals/group. *p < 0.05, **p < 0.01, ***p < 0.001 versus YS. ϕp < 0.05 versus YC. On the right, representative immunoblots for the protein levels of mGluR5, Homer1b/1c, NR1, NR2A, and NR2B are shown
A one-way ANOVA showed significant changes during forced cocaine abstinence in mGluR5 protein expression in the nucleus accumbens (F(2,23) = 9.637, p = 0.0012) and dorsal striatum (F(2,23) = 7.962, p = 0.0036). The posthoc analyses revealed a 45 % rise in mGluR5 protein expression in the nucleus accumbens (p < 0.01 in comparison with yoked saline group, and p < 0.05 in comparison with yoked cocaine group) (Fig. 7b). On the other hand, in the dorsal striatum of animals extinguished form cocaine self-administration, a 47 % decrease in mGluR5 protein expression (p < 0.05) in comparison with saline group and p < 0.01 with yoked cocaine group were detected (Fig. 8b).
Effects of cocaine self-administration (a) and extinction training (b) on the mGluR5, Homer1b/1c, and NMDA (subunits: NR1, NR2A, NR2B) receptor protein expression in the rat nucleus accumbens with a “yoked” control procedure. Data were normalized to saline-treated animals (% of yoked saline; YS) and represent the mean (±SEM) of 8–10 animals/group. **p < 0.01, ***p < 0.001 versus YS; ϕp < 0.05 versus YC. On the right, representative immunoblots for the protein levels of mGluR5, Homer1b/1c, NR1, NR2A, and NR2B are shown
Effects of cocaine self-administration (a) and extinction training (b) on the mGluR5, Homer1b/1c, and NMDA (subunits: NR1, NR2A, NR2B) receptor protein expression in the rat dorsal striatum with a “yoked” control procedure. Data were normalized to saline-treated animals (% of yoked saline; YS) and represent the mean (±SEM) of 8–10 animals/group. *p < 0.05 versus YS; ϕp < 0.05, ϕϕp < 0.01 versus YC. On the right, representative immunoblots for the protein levels of mGluR5, Homer1b/1c, NR1, NR2A, and NR2B are shown
NMDA Subunit Expression
A one-way ANOVA analysis showed that cocaine self-administration resulted in changes in GluN1 protein expression in the hippocampus (F(2,23) = 6.484, p = 0.0064) (Fig. 6a) and in GluN2A protein level in the dorsal striatum (F(2,23) = 4.591, p = 0.0222) (Fig. 8a). The posthoc analyses revealed in rats self-administered cocaine a significant increase in GluN1 subunit protein expression (p < 0.05) in the hippocampus compared to yoked saline and yoked cocaine groups and in the dorsal striatum in GluN2A subunit protein expression (p < 0.05) compared to yoked saline group. Moreover, increasing trends were seen for GluN1, GluN2A-2B subunits in the nucleus accumbens, and in the dorsal striatum for GluN2B subunits (Figs. 7a, 8a).
In animals that extinguished from cocaine active and yoked administration, we found significant changes in NMDA receptor subunits, with more intensive alterations being observed for rats that self-administered cocaine. In those animals, GluN1 subunit expression was elevated in the hippocampus (ANOVA: F(2,23) = 4.966, p = 0.0171; posthoc test: p < 0.05 versus yoked saline group) (Fig. 6b) and the nucleus accumbens (ANOVA: F(2,23) = 71.42, p < 0.0001; posthoc test p < 0.001 versus saline group) (Fig. 7b), while in the prefrontal cortex some trends were observed. In the dorsal striatum, we found a significant decrease of GluN1 subunit protein expression in yoked cocaine group (ANOVA: F(2,23) = 8.245, p = 0.0023; posthoc test p < 0.05 in comparison with yoked saline group and p < 0.01 in comparison with yoked cocaine group) (Fig. 8b).
In animals that extinguished from cocaine self-administration, we report a significant rise (ca. 20 %) of GluN2A subunit expression in the hippocampus (ANOVA: F(2,23) = 5.098, p = 0.0157; posthoc test: p < 0.05) (Fig. 6b), while all rats treated with cocaine showed an enhancement (>50 %) in the prefrontal GluN2A subunit expression (ANOVA: F(2,23) = 14.14, p = 0.0001; posthoc test: at least p < 0.01 in comparison with yoked saline group) (Fig. 5b).
For GluN2B subunit protein expression, we found a significant (ca. 45 %, p < 0.05) rise in the hippocampus of animals withdrawn from cocaine self-administration and a non-significant increase (ca. 27 %) in yoked cocaine controls (ANOVA: F(2,23) = 4.584, p = 0.0223) (Fig. 6b).
Homer1b/1c Expression
As shown in Fig. 8b, only extinction training in rats previously self-administered cocaine produced a 50 % drop in Homer1b/1c protein expression in the dorsal striatum (ANOVA: F(2,23) = 4.514, p = 0.0234; posthoc test: p < 0.05 versus yoked saline and yoked cocaine groups) (Fig. 8b).
Discussion
Our results show alterations in mGluR5 and NMDAR Protein expression following cocaine and its withdrawal in the rat brain that depends on the type of drug delivery and its withdrawal as well as on brain region.
Effects of Contingent Cocaine Delivery on mGluR5 and NMDAR Protein Expression
Cocaine self-administration increased GluN1 and GluN2A subunit expression in the hippocampus and dorsal striatum, respectively. Marked rises were found also for mGluR5 receptor expression in striatum in a way that was independent from the modality of cocaine exposure.
Some previous reports indicate that long-term cocaine self-administration in rats produced an up-regulation of GluN1 gene expression in some limbic and subcortical areas (Crespo et al. 2002), while significant increases in the accumbal GluN1 subunit and a trend in mGluR5 were noticed in cocaine overdose victims as well as in rhesus monkeys that self-administered cocaine for 18 months (Hemby et al. 2005). As increases in the hippocampal, dorsal striatal (present study), and accumbal (Fitzgerald et al. 1996) GluN1 and GluN2A subunits have not been seen after passive repeated cocaine administration, the findings may suggest them as molecular mediators of motivational or/and conditioned aspects of drug abuse. In fact, elevated GluN1 and GluN2 subunit levels may represent an initial step in synaptic plasticity associated with cocaine self-administration. From a functional perspective, both subunits are required for the long-term synaptic changes as GluN1 subunit controls Ca2+ permeability (Huang et al. 2011), while GluN2 subunits promote LTP (Jin and Feig 2010). Preclinical data on rodents show that NMDAR in the hippocampus is involved in the formation of aversive memory (Bonini et al. 2011), while those localized in striatal areas contribute to the memorization of a complex motor task in which the GluN2A subunits have a critical role in the slow acquisition phase of motor learning (Lemay-Clermont et al. 2011). Further research is warranted to examine whether changes observed in the present paper indicate any type of plasticity.
As found, cocaine delivery evoked increases in mGluR5 protein expression. Since we did not address cell surface or intracellular mGluR5 localization (Purgert et al. 2014), we also can only speculate about the functional outcomes of such increases. One possibility is that repeated cocaine via mGluR5 might alter protein-synthesis-dependent LTD (by intracellular mGluR5) or both LTD and LTP (via cell surface mGluR5). Another possibility is that, cocaine pharmacological mechanisms trigger the increase in mGluR5 availability that reflects—among others—the attention and/or the vigilance of animals. In fact, the density of these receptors is reduced in the brain of patients with depression, particularly in brain structures involved in regulating wakefulness (Deschwanden et al. 2011).
The observed changes in Glu receptor protein expression are probably caused by several mechanisms. Among others, former activation of synaptic DA-ergic, NA-ergic, and 5-HT-ergic transmissions via cocaine’s inhibition of the monoamine transporters may be considered (Gatley et al. 1999; Brown et al. 2001). As reported by our group (Wydra et al. 2013) and other teams (Pettit and Justice 1989; Lecca et al. 2007; Parsons et al. 1996) cocaine self-administered or delivered by yoked passive injections potently increases in vivo extracellular DA and/or 5-HT in the nucleus accumbens. By using genetic and pharmacological tools it was shown that the function and subunit composition of NMDARs are highly influenced by monoamine neurotransmitters (Boyer et al. 1998; Masuko et al. 2004; Yuen et al. 2005; Singh et al. 2013). Increased hippocampal GluN1 as well as striatal GluN2A and mGluR5 subunits may also represent a compensatory mechanism to offset the changes in Glu levels. Small increases at a few isolated time points during the self-administration sessions (Sizemore et al. 2000; see also Wydra et al. 2013) as well as decreases in basal Glu extracellular levels of nucleus accumbens in the same behavioral procedure used in the present study were noticed. The engagement of NMDAR and mGluR5 to the rewarding effects of cocaine supports preclinical pharmacological analyses (for review see Pomierny-Chamiolo et al. 2014).
mGluR5 and NMDAR Protein Expression Following Extinction Training
Extinction training evoked significant up-regulation of GluN2A in the prefrontal cortex, GluN1 in the hippocampus and nucleus accumbens, while mGluR5 was either up-regulated in the hippocampus or down-regulated in the prefrontal cortex. These changes appeared in parallel in cocaine self-administered or yoked cocaine animals. Interestingly, only rats with previous voluntary access to cocaine showed increased GluN2A and GluN2B subunit expression in the hippocampus and mGluR5 in the nucleus accumbens; however, the most eminent change in those rats was the reduction in mGluR5 protein level in the striatum. The latter reduction was associated with the Homer1b/1c down-regulation.
Consistent with Ghasemzadeh et al. (2011), a decrease in prefrontal mGluR5 protein expression may reflect a molecular correlate of relapse liability and a trigger to reinstate drug-seeking behavior. As confirmed by pharmacological and genetic analyses, the receptor constitutive activity appears to protect against stress, cue, or drug priming effect during cocaine seeking in rodents or squirrel monkeys (for review see: Pomierny-Chamiolo et al. 2014; Martin-Fardon et al. 2009; Lee et al. 2005; Iso et al. 2006; Kumaresan et al. 2009; Novak et al. 2010). Moreover, the receptor pharmacological blockade in the ventromedial prefrontal cortex reduced, while local mGluR5 stimulation facilitated, extinction learning in cocaine-withdrawn rats (Ben-Shahar et al. 2013). A recent neuroimaging report also indicated a significant reduction in mGluR5 binding in the gray matter of human smokers (Akkus et al. 2013). We also demonstrated a drop in mGluR5 protein expression in the prefrontal cortex of yoked cocaine animals which were exposed to cues and—despite the possibility to associate the drug cue to the operant response—they formed a Pavlovian association between the cue presentation and cocaine effects which were messed during animals’ exposure to experimental chambers in the absence of cocaine and its cue. As no changes in mGluR5 protein or mRNA were detected in cocaine-withdrawn rats left in home cage without extinction training (Ghasemzadeh et al. 2011; Ben-Shahar et al. 2009), these data speak against the pharmacological properties of cocaine or drug motivation (see Twining et al. 2009).
We also show that cocaine withdrawal resulted in a rise in GluN2A subunit expression (without significant changes in GluN1 or GluN2B protein levels) in the prefrontal cortex which seems to be attributable to the previous drug exposure. So far, similar increase was demonstrated after non-contingent cocaine administration in rats (Ary and Szumlinski 2007) and such overexpression of GluN2A subunit finding may suggest that the NMDA receptor kinetics of currents deactivation are faster than in controls (Yashiro and Philpot 2008). Such changes may also be associated with memory impairment (Jacobs and Tsien 2014) or enhanced cue and contextual fear conditioning (Gilmartin et al. 2013).
There was a significant (>50 %) cocaine-dependent increase in mGluR5 protein level in the rat hippocampus at 10-day withdrawal and this is the first report showing changes in protein levels in the rat hippocampus after extinction from cocaine iv delivery. In parallel to mGluR5, we observed a significant elevation of GluN1 subunit level in both cocaine groups, while GluN2A and GluN2B protein expression reached level of significance only in rats that previously self-administered cocaine. As recently shown, GluN1-2A-containing NMDA receptors mediate the formation of lasting contextual and trace fear memory and are more prominent to induce LTP in pyramidal neurons of the CA1 hippocampus (Jin and Feig 2010), while GluN1-2B-containing NMDA receptors are required for mediation trace fear conditioning (Gao et al. 2010). At the same time, 2-week cocaine forced abstinence inhibited proliferation in hippocampal cells (Yamaguchi et al. 2002; Garcia-Fuster et al. 2011) what may be directly linked with local reduction in Glu transmission (Sultan et al. 2013). In other words, changes in hippocampal mGluR5 and GluN1 proteins and in GluN2A and GluN2B subunits may reflect compensatory mechanisms of cocaine-mediated disturbed neurogenesis and memory processes in hippocampal cells.
Increased accumbal mGluR5 and GluN1 (but not GluN2A and GluN2B) receptor expression following extinction training was another finding in this study. Extinction from cocaine self-administration reorganized postsynaptic mGluR5 receptors in the nucleus accumbens subregions (not dissected in the present paper) with reduction in the accumbal shell (Ghasemzadeh et al. 2009c) but no changes in the accumbal core (Knackstedt et al. 2013) or raised (Ghasemzadeh et al. 2009b) after long access (6-hr daily) to cocaine self-administration. Neuroanatomical analyses with local microinfusions confirm the significance of mGluR5 receptor activity localized to accumbal core in controlling cocaine seeking (Backstrom and Hyytia 2007; Wang et al. 2013). A decline in accumbal (Swanson et al. 2001; Hao et al. 2010; Huang et al. 2011) mGluR5 protein expression was seen after withdrawal from non-contingent repeated administration of cocaine in rodents, similar to what others reported following a single passive administration of cocaine (Fourgeaud et al. 2004). Whether such increase reflects new learning processes due to previous unreinforced cocaine administration that occurred in those animals (see above) needs to be determined in additional studies.
The present paper found around a 200 % enhancement in the accumbal GluN1 protein expression after 10-day extinction training sessions and this increase was response-independent and is in line with past studies reporting non-contingent cocaine administration (Scheggi et al. 2002; Schumann and Yaka 2009). The nature of the relationship between GluN1 receptor state-dependent plasticity and response output (incubation?) during cocaine extinction is unresolved (see: Ghasemzadeh et al. 2009c). The lack of significant changes in striatal GluN2 and GluN2B subunit expression due to cocaine self-administration and extinction training extends recent findings in animals with short and long access to self-administered cocaine followed by 10-day home-cage withdrawal (Ben-Shahar et al. 2009).
As shown in this study, extinction training evoked a significant reduction in mGluR5 and GluN1 protein expression, with no changes in GluN2A and GluN2B subunits, in the dorsal striatum of rats with previous cocaine self-administration, but not in yoked cocaine animals. A drop in striatal mGluR5 expression during extinction learning was demonstrated through molecular (reduction in mGluR5 surface expression) and pharmacological (mGluR5 receptor ligands) analyses in rats that previously underwent cocaine self-administration (Knackstedt et al. 2013). At the same time, non-contingent repeated cocaine administration increased mGluR5 mRNA levels and/or protein expression (Ghasemzadeh et al. 1999; Lee et al. 2008) or did not alter NMDA receptor subunits (GluN1, GluN2A, and GluN2B) (Yamamoto and Zahniser 2012) in the rat dorsal striatum. It is not answered whether the observed molecular processes of the Glu signaling at mGluR5 and NMDA receptors in the dorsal striatum are directly linked to messed contextual encoding of extinction or enhanced extinction consolidation.
We also found altered expression (20 % decrease) of the scaffolding protein Homer1b/1c in the dorsal striatum of rats that underwent extinction training after cocaine self-administration. Of note, the same group of animals shows also a potent reduction in striatal mGluR5. Significant changes in striatal Homer1b/1c proteins during extinction from cocaine self-administration were observed previously under different experimental conditions (Ghasemzadeh et al. 2009c; Ben-Shahar et al. 2009) and such consistent changes may indicate extinction training as a trigger of Glu plasticity in the dorsal striatum, a structure critical for the learning and maintenance of goal-directed responding (Koob and Swerdlow 1988). Extinction training disrupts previously learned contextual encoding of extinction or enhances extinction consolidation (cf. Torregrossa et al. 2013); whether or not these processes offer a strategy to reduce cocaine relapse remains to be determined.
Withdrawal from cocaine induces decrease in basal synaptic Glu levels (e.g., Wydra et al. 2013) and in mechanisms responsible for Glu clearance that is different in various brain structures (see Baker et al. 2002; Melendez et al. 2005; Cavelier and Attwell 2005, Madayag et al. 2007, Pendyam et al. 2009; Knackstedt et al. 2010). It is still unknown whether differences in Glu levels and region-dependent mechanisms maintaining the neurotransmitter level are responsible for the changes in receptor expression observed in the present paper following cocaine self-administration. The total receptor amount, which is determined by a balance of protein synthesis (enhanced transcription and translation) and degradation (e.g., ubiquitination and proteasome-mediated degradation) was not addressed in this study as well as we also cannot answer if the labeled proteins were localized to the membrane surface.
Summary
Our results showed that cocaine self-administration and its extinction training produce numerous alterations in mGluR5, NMDA, and Homer1b/1c protein expression that are region specific and are dependent of the manner (contingent or non-contingent) of cocaine administration. Extinction training procedure by withholding cocaine injections and cue-contingent presentations led to diminishment in the active lever pressing that paralleled an increase in the hippocampal GluN2A and B subunit and in the accumbal mGluR5. The latter procedures provoked also simultaneous decrease in mGluR5 and Homer1b/1c protein expression in the dorsal striatum but in other structures such correlation was not detected. These results do not also show correlation between changes in mGluR5 and NMDA protein expression via Homer1b/1c protein.
Abbreviations
- EMCDDA:
-
European Monitoring Center for Drugs and Drug Addiction
- UNDCP:
-
United Nations Office on Drugs and Crime
- NMDAR:
-
N-methyl-D-aspartate receptors
- Glu:
-
Glutamate
- LTP:
-
Long-term potentiation
- LTD:
-
Long-term depression
- FR:
-
Fixed ratio
- DA:
-
Dopamine
- NA:
-
Norepinephrine
- 5-HT:
-
Serotonin
References
Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D et al (2013) Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc Natl Acad Sci USA 110(2):737–742
Alagarsamy S, Saugstad J, Warren L, Mansuy IM, Gereau RW 4th, Conn PJ (2005) NMDA-induced potentiation of mGluR5 is mediated by activation of protein phosphatase 2B/calcineurin. Neuropharmacology 49(Suppl 1):135–145
Anwyl R (1999) Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 29(1):83–120
Ary AW, Szumlinski KK (2007) Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: a two-species comparison. Brain Res 1184:295–305
Backstrom P, Hyytia P (2007) Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology 192(4):571–580
Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW (2002) The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 22(20):9134–9141
Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL et al (2009) Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse 63(7):598–609
Ben-Shahar O, Sacramento AD, Miller BW, Webb SM, Wroten MG, Silva HE et al (2013) Deficits in ventromedial prefrontal cortex group 1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci 33(2):495–506
Bonini JS, Da Silva WC, Da Silveira CK, Kohler CA, Izquierdo I, Cammarota M (2011) Histamine facilitates consolidation of fear extinction. Int J Neuropsychopharmacol 14(9):1209–1217
Boyer PA, Skolnick P, Fossom LH (1998) Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci 10(3):219–233
Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S et al (2005) Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 310(5752):1340–1343
Brown JM, Hanson GR, Fleckenstein AE (2001) Regulation of the vesicular monoamine transporter-2: a novel mechanism for cocaine and other psychostimulants. J Pharmacol Exp Ther 296(3):762–767
Brown AL, Flynn JR, Smith DW, Dayas CV (2011) Down-regulated striatal gene expression for synaptic plasticity-associated proteins in addiction and relapse vulnerable animals. Int J Neuropsychopharmacol 14(8):1099–1110
Bystrowska B, Smaga I, Frankowska M, Filip M (2014) Changes in endocannabinoid and N-acylethanolamine levels in rat brain structures following cocaine self-administration and extinction training. Prog Neuropsychopharmacol Biol Psychiatry 50:1–10
Cavelier P, Attwell D (2005) Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J Physiol 564(Pt 2):397–410
Cornish JL, Duffy P, Kalivas PW (1999) A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience 93(4):1359–1367
Crespo JA, Oliva JM, Ghasemzadeh MB, Kalivas PW, Ambrosio E (2002) Neuroadaptive changes in NMDAR1 gene expression after extinction of cocaine self-administration. Ann N Y Acad Sci 965:78–91
Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A et al (2011) Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry 168(7):727–734
Ferraguti F, Shigemoto R (2006) Metabotropic glutamate receptors. Cell Tissue Res 326(2):483–504
Ferraguti F, Crepaldi L, Nicoletti F (2008) Metabotropic glutamate 1 receptor: current concepts and perspectives. Pharmacol Rev 60(4):536–581
Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ (1996) Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci 16(1):274–282
Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ (2004) A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci 24(31):6939–6945
Gao C, Gill MB, Tronson NC, Guedea AL, Guzman YF, Huh KH et al (2010) Hippocampal NMDA receptor subunits differentially regulate fear memory formation and neuronal signal propagation. Hippocampus 20(9):1072–1082
Garcia-Fuster MJ, Flagel SB, Mahmood ST, Mayo LM, Thompson RC, Watson SJ et al (2011) Decreased proliferation of adult hippocampal stem cells during cocaine withdrawal: possible role of the cell fate regulator FADD. Neuropsychopharmacology 36(11):2303–2317
Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS et al (1999) Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology 146(1):93–100
Ghasemzadeh MB, Nelson LC, Lu XY, Kalivas PW (1999) Neuroadaptations in ionotropic and metabotropic glutamate receptor mRNA produced by cocaine treatment. J Neurochem 72(1):157–165
Ghasemzadeh MB, Mueller C, Vasudevan P (2009a) Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience 159(1):414–426
Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR (2009b) Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci Lett 452(2):167–171
Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR (2009c) Region specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res 1267:89–102
Ghasemzadeh MB, Vasudevan P, Giles C, Purgianto A, Seubert C, Mantsch JR (2011) Glutamatergic plasticity in medial prefrontal cortex and ventral tegmental area following extended-access cocaine self-administration. Brain Res 1413:60–71
Gilmartin MR, Kwapis JL, Helmstetter FJ (2013) NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learn Mem 20(6):290–294
Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW (2013) Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron 77(5):867–872
Hao Y, Martin-Fardon R, Weiss F (2010) Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry 68(3):240–248
Hemby SE, Horman B, Tang W (2005) Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain Res 1064(1–2):75–82
Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH et al (2011) Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci 31(11):4194–4203
Iso Y, Grajkowska E, Wroblewski JT, Davis J, Goeders NE, Johnson KM et al (2006) Synthesis and structure-activity relationships of 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine analogues as potent, noncompetitive metabotropic glutamate receptor subtype 5 antagonists; search for cocaine medications. J Med Chem 49(3):1080–1100
Jacobs SA, Tsien JZ (2014) Overexpression of the NR2A subunit in the forebrain impairs long-term social recognition and non-social olfactory memory. Genes Brain Behav 13(4):376–384
Jin SX, Feig LA (2010) Long-term potentiation in the CA1 hippocampus induced by NR2A subunit-containing NMDA glutamate receptors is mediated by Ras-GRF2/Erk map kinase signaling. PLoS One 5(7):e11732
Kalivas PW (2004) Glutamate systems in cocaine addiction. Curr Opin Pharmacol 4(1):23–29
Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10(8):561–572
Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O et al (2010) Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science 328(5986):1709–1712
Keck TM, Zou MF, Bi GH, Zhang HY, Wang XF, Yang HJ et al (2014) A novel mGluR5 antagonist, MFZ 10-7, inhibits cocaine-taking and cocaine-seeking behavior in rats. Addict Biol 19(2):195–209
Kim JH, Lawrence AJ (2014) Drugs currently in Phase II clinical trials for cocaine addiction. Expert Opin Investig Drugs 23(8):1105–1122
Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW (2010) Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci 30(23):7984–7992
Knackstedt LA, Trantham-Davidson HL, Schwendt M (2013) The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addict Biol 19(1):87–101
Koob GF, Swerdlow NR (1988) The functional output of the mesolimbic dopamine system. Ann N Y Acad Sci 537:216–227
Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD et al (2009) Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res 202(2):238–244
Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G (2007) Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology 191(3):653–667
Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD (2005) Attenuation of behavioral effects of cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther 312(3):1232–1240
Lee DK, Bian S, Rahman MA, Shim YB, Shim I, Choe ES (2008) Repeated cocaine administration increases N-methyl-d-aspartate NR1 subunit, extracellular signal-regulated kinase and cyclic AMP response element-binding protein phosphorylation and glutamate release in the rat dorsal striatum. Eur J Pharmacol 590(1–3):157–162
Lemay-Clermont J, Robitaille C, Auberson YP, Bureau G, Cyr M (2011) Blockade of NMDA receptors 2A subunit in the dorsal striatum impairs the learning of a complex motor skill. Behav Neurosci 125(5):714–723
Low CM, Wee KS (2010) New insights into the not-so-new NR3 subunits of N-methyl-D-aspartate receptor: localization, structure, and function. Mol Pharmacol 78(1):1–11
Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M et al (2007) Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci 27(51):13968–13976
Madhavan A, Argilli E, Bonci A, Whistler JL (2013) Loss of D2 dopamine receptor function modulates cocaine-induced glutamatergic synaptic potentiation in the ventral tegmental area. J Neurosci 33(30):12329–12336
Martin-Fardon R, Baptista MA, Dayas CV, Weiss F (2009) Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther 329(3):1084–1090
Masuko T, Suzuki I, Kizawa Y, Kusama-Eguchi K, Watanabe K, Kashiwagi K et al (2004) Monoamines directly inhibit N-methyl-D-aspartate receptors expressed in Xenopus oocytes in a voltage-dependent manner. Neurosci Lett 371(1):30–33
McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23(8):3531–3537
Melendez RI, Vuthiganon J, Kalivas PW (2005) Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther 314(1):139–147
Novak M, Halbout B, O’Connor EC, Rodriguez Parkitna J, Su T, Chai M et al (2010) Incentive learning underlying cocaine-seeking requires mGluR5 receptors located on dopamine D1 receptor-expressing neurons. J Neurosci 30(36):11973–11982
Parsons LH, Koob GF, Weiss F (1996) Extracellular serotonin is decreased in the nucleus accumbens during withdrawal from cocaine self-administration. Behav Brain Res 73(1–2):225–228
Paxinos G, Watson C (1998) The rat brain in stereotaxic coor-dinates, 4th edn. American Press Inc, San Diego
Pendyam S, Mohan A, Kalivas PW, Nair SS (2009) Computational model of extracellular glutamate in the nucleus accumbens incorporates neuroadaptations by chronic cocaine. Neuroscience 158(4):1266–1276
Pettit HO, Justice JB Jr (1989) Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav 34(4):899–904
Pomierny-Chamiolo L, Moniczewski A, Wydra K, Suder A, Filip M (2013) Oxidative stress biomarkers in some rat brain structures and peripheral organs underwent cocaine. Neurotox Res 23(1):92–102
Pomierny-Chamiolo L, Rup K, Pomierny B, Niedzielska E, Kalivas PW, Filip M (2014) Metabotropic glutamatergic receptors and their ligands in drug addiction. Pharmacol Ther 142(3):281–305
Purgert CA, Izumi Y, Jong YJ, Kumar V, Zorumski CF, O’Malley KL (2014) Intracellular mGluR5 can mediate synaptic plasticity in the hippocampus. J Neurosci 34(13):4589–4598
Ronesi JA, Huber KM (2008) Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci 28(2):543–547
Scheggi S, Mangiavacchi S, Masi F, Gambarana C, Tagliamonte A, De Montis MG (2002) Dizocilpine infusion has a different effect in the development of morphine and cocaine sensitization: behavioral and neurochemical aspects. Neuroscience 109(2):267–274
Schumann J, Yaka R (2009) Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci 29(21):6955–6963
Shen H, Kalivas PW (2013) Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration. Int J Neuropsychopharmacol 16(5):1165–1167
Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW (2009) Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci 29(9):2876–2884
Singh C, Bortolato M, Bali N, Godar SC, Scott AL, Chen K et al (2013) Cognitive abnormalities and hippocampal alterations in monoamine oxidase A and B knockout mice. Proc Natl Acad Sci USA 110(31):12816–12821
Sizemore GM, Co C, Smith JE (2000) Ventral pallidal extracellular fluid levels of dopamine, serotonin, gamma amino butyric acid, and glutamate during cocaine self-administration in rats. Psychopharmacology 150(4):391–398
Sultan KT, Brown KN, Shi SH (2013) Production and organization of neocortical interneurons. Front Cell Neurosci 7:221
Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW (2001) Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci 21(22):9043–9052
Szumlinski KK, Ary AW, Lominac KD (2008) Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol 75(1):112–133
Torregrossa MM, Gordon J, Taylor JR (2013) Double dissociation between the anterior cingulate cortex and nucleus accumbens core in encoding the context versus the content of pavlovian cocaine cue extinction. J Neurosci 33(19):8370–8377
Twining RC, Bolan M, Grigson PS (2009) Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behav Neurosci 123(4):913–925
Tzschentke TM, Schmidt WJ (2003) Glutamatergic mechanisms in addiction. Mol Psychiatry 8(4):373–382
Volkow ND, Fowler JS, Wang GJ (2003) Positron emission tomography and single-photon emission computed tomography in substance abuse research. Semin Nucl Med 33(2):114–128
Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW (2013) Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol 18(1):40–49
Wydra K, Golembiowska K, Zaniewska M, Kaminska K, Ferraro L, Fuxe K et al (2013) Accumbal and pallidal dopamine, glutamate and GABA overflow during cocaine self-administration and its extinction in rats. Addict Biol 18(2):307–324
Yamaguchi M, Suzuki T, Abe S, Hori T, Kurita H, Asada T et al (2002) Repeated cocaine administration differentially affects NMDA receptor subunit (NR1, NR2A-C) mRNAs in rat brain. Synapse 46(3):157–169
Yamamoto DJ, Zahniser NR (2012) Differences in rat dorsal striatal NMDA and AMPA receptors following acute and repeated cocaine-induced locomotor activation. PLoS One 7(5):e37673
Yashiro K, Philpot BD (2008) Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 55(7):1081–1094
Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z (2005) Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci 25(23):5488–5501
Acknowledgments
This study was supported by the Grant NN40 545340 from the Ministry of Science and Higher Education (Warszawa, Poland).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Pomierny-Chamiolo, L., Miszkiel, J., Frankowska, M. et al. Withdrawal from Cocaine Self-administration and Yoked Cocaine Delivery Dysregulates Glutamatergic mGlu5 and NMDA Receptors in the Rat Brain. Neurotox Res 27, 246–258 (2015). https://doi.org/10.1007/s12640-014-9502-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-014-9502-z