Abstract
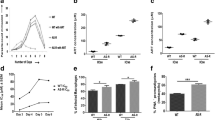
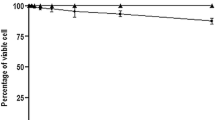
Visceral leishmaniasis (kala-azar), a life threatening disease caused by L. donovani, is a latent threat to more than 147 million people living in disease endemic South East Asia region of the Indian subcontinent. The therapeutic option to control leishmanial infections are very limited, and at present comprise only two drugs, an antifungal amphotericin B and an antitumor miltefosine, which are also highly vulnerable for parasitic resistance. Therefore, identification and development of alternate control measures is an exigent requirement to control leishmanial infections. In this study, we report that functionally induced expression of solute carrier protein family 11 member 1 (Slc11a1), a transmembrane divalent cationic transporter recruited on the surface of phagolysosomes after phagocytosis of parasites, effectively inhibits Leishmania donovani growth in host macrophages. Further, the increased Slc11a1 functionality also resulted in increased production of NOx, TNF-α and IL-12 by activated macrophages. The findings of this study signify the importance of interplay between Slc11a1 expression and macrophages activation that can be effectively used to control of Leishmania growth and survival.



Similar content being viewed by others
References
Appelberg R (2006) Macrophage nutriprive antimicrobial mechanisms. J Leukoc Biol 79:1117–1128
Balanco JM, Pral EM, da Silva S, Bijovsky AT, Mortara RA, Alfieri SC et al (1998) Axenic cultivation and partial characterization of Leishmania braziliensis amastigote-like stages. Parasitology 116:103–113
Blackwell JM, Searle S, Mohamed H, White JK (2003) Divalent cation transport and susceptibility to infectious and autoimmune disease: continuation of the Ity/Lsh/Bcg/Nramp1/Slc11a1 gene story. Immunol Lett 85:197–203
Cabantchik ZI, Glickstein H, Milgram P, Breurer W (1996) A fluorescence assay for assessing chelation of intracellular iron in a membrane model system and in mammalian cells. Anal Biochem 233:221–227
Caron J, Lariviere L, Nacache M, Tam M, Stevenson MM, McKerly C et al (2006) Influence of Slc11a1 on the outcome of Salmonella enterica serovar Enteritidis infection in mice is associated with Th polarization. Infect Immun 74:2787–2802
Castellanos-Serra LR, Fernandez-Patron C, Hardy E, Santana H, Huerta V (1997) High yield elution of proteins from sodium dodecyl sulfate-polyacrylamide gels at the low-picomole level. Application to N-terminal sequencing of a scarce protein and to in-solution biological activity analysis of on-gel renatured proteins. J Protein Chem 16:415–419
Cellier MF, Courville P, Campion C (2007) Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect 9:1662–1670
Chakraborty R, Mukherjee S, Basu MK (1996) Oxygen-dependent leishmanicidal activity of stimulated macrophages. Mol Cell Biochem 154:23–29
Chakravarty J, Sundar S (2010) Drug resistance in leishmaniasis. J Glob Infect Dis 2:167–176
Dai YD, Marrero IG, Gros P, Zaghouani H, Wicker LS, Sercarz EE (2009) Slc11a1 enhances the autoimmune diabetogenic T-cell response by altering processing and presentation of pancreatic islet antigens. Diabetes 58:156–164
Das NK, Biswas S, Solanki S, Mukhopadhyay CK (2009) Leishmania donovani depletes labile iron pool to exploit iron uptake capacity of macrophage for its intracellular growth. Cell Microbiol 11:83–94
Dempsey LA (2013) Metabolic control of cytokines. Nat Immunol 14:776
Forbes JR, Gros P (2001) Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol 9:397–403
Freitas-Junior LH, Chatelain E, Kim HA, Siqueira-Neto JL (2012) Visceral leishmaniasis treatment: what do we have, what do we need and how to deliver it? Int J Parasitol Drugs Drug Resist 2:11–19
Fritsche G, Dlaska M, Barton H, Theurl I, Garimorth K, Weiss G (2003) Nramp1 functionality increases inducible nitric oxide synthase transcription via stimulation of IFN regulatory factor 1 expression. J Immunol 171:1994–1998
Fritsche G, Nairz M, Theurl I, Mair S, Bellmann-Weiler R, Barton HC et al (2007) Modulation of macrophage iron transport by Nramp1 (Slc11a1). Immunobiology 212:751–757
Fritsche G, Nairz M, Werner ER, Barton HC, Weiss G (2008) Nramp1-functionality increases iNOS expression via repression of IL-10 formation. Eur J Immunol 38:3060–3067
Fritsche G, Nairz M, Libby SJ, Fang FC, Weiss G (2012) Slc11a1 (Nramp1) impairs growth of Salmonella enterica serovar typhimurium in macrophages via stimulation of lipocalin-2 expression. J Leukoc Biol 92:353–359
Ghasemi A, Zahedi Asl S, Mehrabi Y, Saadat N, Azizi F (2008) Serum nitric oxide metabolite levels in a general healthy population: relation to sex and age. Life Sci 83:326–331
Gruenheid S, Cellier M, Vidal S, Gros P (1995) Identification and characterization of a second mouse Nramp gene. Genomics 25:514–525
Gupta G, Oghumu S, Satoskar AR (2013) Mechanisms of immune evasion in leishmaniasis. Adv Appl Microbiol 82:155–184
Huynh C, Andrews NW (2008) Iron acquisition within host cells and the pathogenicity of Leishmania. Cell Microbiol 10:293–300
Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P (2000) Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med 192:1237–1248
Kane MM, Mosser DM (2001) The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol 166:1141–1147
Liese J, Schleicher U, Bogdan C (2008) The innate immune response against Leishmania parasites. Immunobiology 213:377–387
Liu D, Uzonna JE (2012) The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front Cell Infect Microbiol 2:83
Nairz M, Fritsche G, Crouch ML, Barton HC, Fang FC, Weiss G (2009) Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol 11:1365–1381
Nylen S, Gautam S (2010) Immunological perspectives of leishmaniasis. J Glob Infect Dis 2:135–146
Prajapati VK, Awasthi K, Yadav TP, Rai M, Srivastava ON, Sundar S (2012) An oral formulation of amphotericin B attached to functionalized carbon nanotubes is an effective treatment for experimental visceral leishmaniasis. J Infect Dis 205:333–336
Purkait B, Kumar A, Nandi N, Sardar AH, Das S, Kumar S et al (2012) Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother 56:1031–1041
Selvapandiyan A, Dey R, Nylen S, Duncan R, Sacks D, Nakhasi HL (2009) Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J Immunol 183:1813–1820
Singh N, Kumar M, Singh RK (2012) Leishmaniasis: current status of available drugs and new potential drug targets. Asian Pac J Trop Med 5:485–497
Singh N, Bajpai S, Kumar V, Gour JK, Singh RK (2013) Identification and functional characterization of Leishmania donovani secretory peroxidase: delineating its role in NRAMP1 regulation. PLoS ONE 8:e53442
Soe-Lin S, Sheftel AD, Wasyluk B, Ponka P (2008) Nramp1 equips macrophages for efficient iron recycling. Exp Hematol 36:929–937
Srivastava A, Singh N, Mishra M, Kumar V, Gour JK, Bajpai S et al (2012) Identification of TLR inducing Th1-responsive Leishmania donovani amastigote-specific antigens. Mol Cell Biochem 359:359–368
Stober CB, Brode S, White JK, Popoff JF, Blackwell JM (2007) Slc11a1, formerly Nramp1, is expressed in dendritic cells and influences major histocompatibility complex class II expression and antigen-presenting cell function. Infect Immun 75:5059–5067
Valdez Y, Diehl GE, Vallance BA, Grassl GA, Guttman JA, Brown NF et al (2008) Nramp1 expression by dendritic cells modulates inflammatory responses during Salmonella Typhimurium infection. Cell Microbiol 10:1646–1661
Weiss G, Werner-Felmayer G, Werner ER, Grunewald K, Wachter H, Hentze MW (1994) Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med 180:969–976
WHO (2005) Regional Strategic Framework for Elimination of Kala-azar from the South-East Asia Region (2005–2015). New Delhi
WHO (2015) Kala-azar elimination programme. Switzerland, Geneva
Acknowledgements
Financial support from Department of Science and Technology, New Delhi (SB/SO/HS/0091/2013) is greatly acknowledged. The authors MGR and NT are extremely thankful to BHU and UGC, New Delhi, respectively for their research fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, N., Gedda, M.R., Tiwari, N. et al. Solute carrier protein family 11 member 1 (Slc11a1) activation efficiently inhibits Leishmania donovani survival in host macrophages. J Parasit Dis 41, 671–677 (2017). https://doi.org/10.1007/s12639-016-0864-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-016-0864-4