Abstract
Background
Intraocular pressure is increased during laparoscopic surgeries performed in a steep Trendelenburg position. This study compared the effects of propofol with those of sevoflurane on intraocular pressure in patients undergoing robot-assisted laparoscopic radical prostatectomy in a 30° Trendelenburg position.
Methods
Sixty-six patients were randomly allocated to a maintenance anesthetic consisting of remifentanil and sevoflurane (Sevoflurane Group) or remifentanil and propofol (Propofol Group). Intraocular pressure (IOP) was measured at nine predefined time points, including baseline (T0), five minutes after establishing pneumoperitoneum (T2), 30 min after establishing the Trendelenburg position with pneumoperitoneum (T3), five minutes after returning to the horizontal position (T4), and immediately after tracheal extubation (T6). The primary outcome was the change in intraocular pressure from T0 to T3.
Results
The results of linear mixed model analysis showed that intraocular pressure differed between the two groups (P = 0.0039). At T3, the mean (SD) IOP was greater in the Sevoflurane Group [23.5 (4.3) mmHg] than in the Propofol Group [19.9 (3.8) mmHg] (P = 0.0019). At T2 and T6, IOP was also greater in the Sevoflurane Group than in the Propofol Group (P = 0.038 and P = 0.009, respectively). There was a statistically significant increase in intraocular pressure from baseline to T3 (pneumoperitoneum and steep Trendelenberg) in the Sevoflurane Group [6.0 (5.0) mmHg; P < 0.001] but not in the Propofol Group [2.1 (5.1) mmHg; P = 0.136]. None of the patients experienced ocular complications.
Conclusions
Intraocular pressure increases after pneumoperitoneum and the steep Trendelenburg position are established. This increase is less with propofol than with sevoflurane anesthesia. This trial was registered at ClinicalTrials.gov: NCT01744262.
Résumé
Contexte
La pression intraoculaire augmente pendant les chirurgies par laparoscopie réalisées en position de Trendelenburg fortement inclinée. Cette étude a comparé les effets du propofol à ceux du sévoflurane sur la pression intraoculaire chez des patients subissant une prostatectomie radicale par laparoscopie assistée par robot dans une position de Trendelenburg inclinée à 30°.
Méthode
Une anesthésie de maintien consistant en rémifentanil et sévoflurane (groupe sévoflurane) ou en rémifentanil et propofol (groupe propofol) a été aléatoirement attribuée à soixante-six patients. La pression intraoculaire (PIO) a été mesurée à neuf moments prédéterminés, notamment au début de l’intervention (T0), cinq minutes après la mise en place du pneumopéritoine (T2), 30 min après le positionnement en position de Trendelenburg avec le pneumopéritoine (T3), cinq minutes après le retour en position horizontale (T4), et immédiatement après l’extubation trachéale (T6). Le critère d’évaluation principal était le changement de pression intraoculaire entre T0 et T3.
Résultats
Les résultats de l’analyse de modèle linéaire mixte ont démontré que la pression intraoculaire différait entre les deux groupes (P = 0,0039). Au point T3, la PIO moyenne (ÉT) était plus élevée dans le groupe sévoflurane [23,5 (4,3) mmHg] que dans le groupe propofol [19,9 (3,8) mmHg] (P = 0,0019). Aux points T2 et T6, la PIO était également plus élevée dans le groupe sévoflurane que dans le groupe propofol (P = 0,038 et P = 0,009, respectivement). Une augmentation statistiquement significative de la pression intraoculaire a été observée entre les mesures au début de l’intervention et celles au point T3 (pneumopéritoine et Trendelenburg fortement inclinée) dans le groupe sévoflurane [6,0 (5,0) mmHg; P < 0,001], mais pas dans le groupe propofol [2,1 (5,1) mmHg; P = 0,136]. Aucun des patients n’a souffert de complications oculaires.
Conclusion
La pression intraoculaire augmente après la mise en place du pneumopéritoine et d’une position de Trendelenburg fortement inclinée. Cette augmentation est moindre avec une anesthésie au propofol qu’avec une anesthésie au sévoflurane. Cette étude est enregistrée sous ClinicalTrials.gov : NCT01744262.
Similar content being viewed by others
Postoperative vision loss (POVL) in the setting of non-ophthalmic surgery is a rare but disastrous event that can be permanently debilitating for the patient. This complication has been reported in patients undergoing surgery in the prone position but also in the steep Trendelenburg position.1–7 The most common type of POVL after non-ocular surgery is known to be ischemic optic neuropathy (ION),5,8 which is thought to be caused by venous congestion of the orbit and decreased ocular perfusion pressure (OPP).3 It is known that increased venous pressure within the eye can possibly lead to an increase in intraocular pressure (IOP), and OPP is estimated as the difference between mean arterial blood pressure and IOP.3 In this sense, elevated IOPs may serve as a surrogate marker of ocular venous congestion and decreased perfusion of the optic nerve. The existing literature indicates that laparoscopic surgery performed with intraperitoneal carbon dioxide (CO2) insufflation results in a typical state of increased IOP.9,10 This increase in IOP is known to be a time-dependent phenomenon which is considerably worsened when combined with a steep Trendelenburg position.1–3,7
Previous studies have reported a decrease in IOP with intravenous hypnotic agents, inhalation anesthetics, and opioids;9,11–15 however, there is controversy regarding the superiority of propofol over inhalation anesthetics in suppressing the increase in IOP. While some reported that propofol was more effective than sevoflurane13 and isoflurane,9 others reported otherwise.14–16 Robot-assisted laparoscopic radical prostatectomy (RALRP) is performed in the steep Trendelenburg position and often requires a prolonged duration of CO2 pneumoperitoneum, which may place the patient at risk for ocular complications due to increased IOP and decreased OPP. This randomized controlled trial was conducted to evaluate and compare the changes in IOP in patients receiving RALRP under general anesthesia with either propofol-based total intravenous anesthesia (TIVA) or inhalation anesthesia with sevoflurane.
Methods
The study protocol was approved by the Institutional Review Board and Hospital Research Ethics Committee of Severance Hospital, Yonsei University Health System, Seoul, Korea on April 28, 2011. All participants were recruited at the anesthesiology preoperative evaluation clinic, and all provided written informed consent. Sixty-six adult patients who were over the age of 50 yr, American Society of Anesthesiologists physical status I or II, and scheduled for RALRP using the da Vinci™ Robot System (Intuitive Surgical, Inc., Mountain View, CA, USA) under general anesthesia were enrolled from May 2011 to March 2012. An ophthalmologist not involved in the study conducted a preoperative evaluation on all patients to measure preoperative IOP, examine the optic fundus, and screen for any corneal abnormalities or ophthalmologic disorders, such as glaucoma, cataract, macular degeneration, and retinopathies. Exclusion criteria included patients with baseline IOPs > 30 mmHg, diabetic retinopathy, cataract, known allergies to anesthetic drugs, uncontrolled hypertension, unstable angina, or congestive heart failure. Patients who had received surgery or medications for treatment of previously diagnosed glaucoma were also excluded. A computer-generated table of random numbers placed in sealed envelopes was used to assign patients immediately and randomly in a 1:1 ratio for parallel treatment arms to either the sevoflurane-based inhalation anesthesia (Sevoflurane Group, n = 33) or the propofol-based TIVA (Propofol Group, n = 33). No stratification or block randomization was done. All study investigators, except the anesthesiologist who took part in the operation, were blinded to group assignment.
On the day of the operation, patients were premedicated with intramuscular midazolam 0.05 mg·kg−1 and glycopyrrolate 0.2 mg at one hour and just before the induction of anesthesia, respectively. The patients arrived at the operating room with a secured intravenous access through which they received lactated Ringer’s solution at a rate of 5 mL·kg−1·hr−1. Infusion rates were tailored to each patient according to intraoperative urine output and blood loss. Standard monitoring devices were applied, and a 20G radial artery catheter was inserted under local anesthesia before induction of anesthesia for continuous blood pressure monitoring and repeated arterial blood gas analysis. Anesthesia was induced with propofol and remifentanil. After loss of consciousness, rocuronium 0.6 mg·kg−1 was administered to facilitate tracheal intubation. Controlled ventilation was performed with 40% oxygen in air to maintain end-tidal CO2 (ETCO2) at 35-40 mmHg, and a positive end-expiratory pressure of 5 cmH2O was applied. Body temperature was maintained at 36-37°C using a forced-air warming system. After induction of anesthesia, a central venous catheter was inserted via the right internal jugular vein for monitoring of central venous pressure (CVP).
A remifentanil infusion was administered in all patients using a target-controlled infusion system (Orchestra® Base Primea, Fresenius Vial, France) according to the Minto model,17 and the effect-site concentration was adjusted in the range of 2-5 ng·mL−1. In the Sevoflurane Group, end-tidal sevoflurane concentrations were maintained at 1.5-2.5% after induction with propofol 1.5 mg·kg−1. In the Propofol Group, propofol was infused using a target-controlled infusion system (Orchestra® Base Primea, Fresenius Vial, France) for induction and maintenance of anesthesia according to the Marsh model,18 and the effect-site concentration was kept in the range of 2-5 μg·mL−1. The anesthetics administered in each group were titrated to maintain mean arterial pressure (MAP) and heart rate within 20% of baseline values and to provide adequate depth of anesthesia. A bispectral index score (BIS) monitor (Aspect A-2000®, Aspect Medical System Inc., Newton, MA, USA) was used, and the BIS was maintained in the range of 40-60. Additional boluses of rocuronium 0.15 mg·kg−1 were given as needed to maintain adequate intraoperative neuromuscular blockade. At the end of surgery, reversal of neuromuscular blockade was performed with neostigmine 50 μg·kg−1 and glycopyrrolate 10 μg·kg−1intravenously. During the operation, CO2 pneumoperitoneum was induced to maintain a mean (SD) intra-abdominal pressure of 15 (5) mmHg using a CO2 insufflator in a 30° Trendelenburg position. The Trendelenburg angle was accurately adjusted by the operating table controller which showed the tilted angle in numbers.
The arterial line transducer was placed at patient eye level and moved as appropriate whenever the patient’s position was changed. Central venous pressure was measured with respect to heart level. Intraocular pressure was measured by a single ophthalmologist using a handheld applanation tonometer (Tono-Pen® XL, Medtronic, Jacksonville, FL, USA); the ophthalmologist was blinded to the anesthetic given. Both MAP and IOP were measured at nine predefined time points (Table 1). The IOP was measured three times at each time point, and the median value was retained for analysis. The primary outcome measurement was the change in IOP from baseline (T0) to 30 min after being positioned in the steep Trendelenburg position with CO2 pneumoperitoneum (T3). For each patient, we recorded whether they experienced an abnormally high IOP at any time. Normal IOP was defined as being < 21 mmHg,19 while IOP ≥ 24 mmHg was defined as the treatment indication of glaucoma.20 Ocular perfusion pressure was calculated as the difference between IOP and MAP measured at eye level.21 Peak inspiratory pressures (PIP), CVP, and end-tidal CO2 (ETCO2) were measured at T1 to T5. Blood was drawn for arterial blood gas analysis at T1, T3, and T5. Recording of vital signs and data analyses were performed by a person who was blinded to patient allocation.
Dichotomous variables are shown as numbers (percentages) and continuous variables are shown as mean (SD). Dichotomous variables were compared using the Chi square test. Repeated measured variables, such as IOP and OPP, were analyzed using a linear mixed model with patient indicator as a random effect and group, time, and group-by-time as fixed effects. The group-by-time interaction assesses whether the change over time differs between groups. Between-group comparisons of continuous variables other than those previously mentioned were performed by Student’s two-sample t test. The changes in IOP at T3 compared with T0 were analyzed with the linear mixed model. Post-hoc analysis with Bonferroni correction was done for multiple comparisons when repeated measured variables showed a significant difference between the two groups. Linear mixed models were used to assess correlation between IOP and PIP, CVP and ETCO2 over the time period T1-T5, and P values were calculated using Fisher’s z transformation. All statistical tests were two-tailed, and P values < 0.05 were considered statistically significant.
The present study was designed to validate the superiority of propofol-based TIVA over sevoflurane anesthesia. In a previous study, the mean (SD) IOP in the propofol-based TIVA group after induction of anesthesia was 6.0 (3.2) mmHg compared with 8.9 (3.4) mmHg in the sevoflurane inhalational anesthesia group.13 To detect a mean (SD) difference in IOP of 2.9 (3.4) mmHg, power estimation analysis suggested that 30 patients per group would be required to obtain a power of 90%, considering a type I error of 0.05. Considering a drop-out rate of 10%, we recruited 33 patients in each group. All statistical analyses were performed using SAS® version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Sixty-seven patients were assessed for eligibility, and one patient was excluded after ophthalmologic evaluation due to senile cataract. The remaining 66 patients were randomized into two groups, and RALRP was performed as planned in all patients. Complete data sets from all 66 patients were analyzed without any missing data. One patient in the Sevoflurane Group and one patient in the Propofol Group were found to have normotensive glaucoma at the preoperative visit to the ophthalmologist. The two patients were advised regarding postoperative follow up; however, they were not excluded from the study. There were no clinically important differences between the two groups in demographic data, duration of anesthesia, pneumoperitoneum and Trendelenburg time, and intraoperative fluid input and output (Table 2). Intraoperative arterial blood gas data were also comparable between the two groups at time points T1, T3, and T5. Mean arterial pressure during the perioperative period, intraoperative CVP, respiratory settings, ETCO2, BIS, and effect-site concentration of remifentanil were similar between the two groups (Table 3).
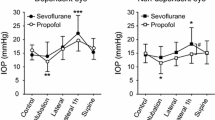
At baseline, IOP was similar in both groups, with mean (SD) values being 17.5 (3.6) mmHg in the Sevoflurane Group and 17.9 (3.7) mmHg in the Propofol Group. Six patients in the Sevoflurane Group and eight patients in the Propofol Group were found to have baseline IOPs > 20 mmHg. After induction of anesthesia, IOP decreased in both groups but increased with the establishment of pneumoperitoneum and Trendelenburg position. Values of IOP returned to baseline at one and 24 hr after surgery (Figure A). During maintenance of anesthesia (from T2 to T6), IOP was greater in the Sevoflurane Group than in the Propofol Group (linear mixed model analysis; P = 0.0039).
In the Sevoflurane Group, IOP was increased significantly at T3 compared with baseline; the difference was 6.0 (5.0) mmHg (P < 0.001). In the Propofol Group, the increase was 2.1 (5.1) mmHg and did not reach statistical significance (P = 0.136). The IOP values at T3 were 23.5 (4.3) mmHg in the Sevoflurane Group and 19.9 (3.8) mmHg in the Propofol Group (difference = 3.5 mmHg; 95% confidence interval [CI]: 1.5 to 5.5; P = 0.0019). The IOP was also greater in the Sevoflurane Group than in the Propofol Group at T2 [18.4 (5.6) mmHg vs 15 (3.4) mmHg, respectively; P = 0.038] and at T6 [21.4 (5.4) vs 17 (4.7) mmHg, respectively; P = 0.009]. Figure A shows the mean IOP values at each time point in each group. Post-hoc analysis with Bonferonni correction revealed that IOP values at T2, T3, and T6 were significantly lower in the Propofol Group compared with the Sevoflurane Group.
Among patients with a normal IOP at baseline (≤ 20 mmHg), IOP was ≥ 24 mmHg at any time point in 20/27 (74%) patients in the Sevoflurane Group and 8/25 (32%) patients in the Propofol Group (difference 42%; 95% CI: 22 to 62; P = 0.002).
A significant difference in OPP was found between the two groups in the linear mixed model analysis (P = 0.047) (Figure B); however, post-hoc analysis with Bonferroni correction revealed no significant difference at any specific time point. Both CVP and PIP were found to be significantly correlated with IOP between T1 and T5 (r = 0.44; 95% CI: 0.21 to 0.62; P = 0.0003, and r = 0.34; 95% CI: 0.1 to 0.54; P = 0.0064, respectively), while ETCO2 was not (r = 0.0816; P = 0.5264). None of the patients experienced ocular complications.
Discussion
In the present study, we found that propofol-based TIVA is more effective than sevoflurane-based inhalation anesthesia in attenuating IOP increase during RALRP with pneumoperitoneum and steep Trendelenberg. Moreover, propofol-based TIVA may be more helpful in maintaining higher OPP during RALRP compared with sevoflurane-based inhalation anesthesia.
In several previous studies, propofol-based TIVA has been reported to be superior to inhalation anesthesia for lowering IOP. Schafer et al. 13 reported that the decrease in IOP during cataract surgery was significantly greater with propofol-based TIVA than with sevoflurane inhalation anesthesia. Moreover, Mowafi et al. 9 found that propofol-based TIVA was superior to isoflurane inhalation anesthesia in attenuating the increase in IOP during laparoscopic surgery in the Trendelenburg position. Nevertheless, despite the significant increase in IOPs observed with isoflurane anesthesia in their study, all absolute values of IOP were found to be within the normal range (< 20 mmHg). In contrast, the mean IOP levels in the present study were > 20 mmHg in the Sevoflurane group during pneumoperitoneum in the Trendelenburg position. This discrepancy between the previous study and the present study seems to be due to the difference in study population. While Mowafi et al. 9 conducted their study in young female patients with a mean age of 31 yr, most patients in the present study were older than 60 yr. In another study by Awad et al.,7 results showed that IOPs increased beyond 20 mmHg in patients undergoing RALRP in the steep Trendeleburg position with desflurane anesthesia. Patients undergoing RALRP are usually elderly, and therefore, they often present with medical comorbidities. Evidence suggests that vascular diseases, such as atherosclerosis, vasospastic diseases, and diabetes, are related to dysfunctional autoregulation of ocular blood flow, likely contributing to glaucomatous optic neuropathy.22 It is well known that even transient episodes of increased IOP may jeopardize retinal perfusion and cause retinal ischemia in older patients with arteriosclerotic involvement of the retinal artery.23 The incidence of undiagnosed glaucoma is also known to increase with age; therefore, the overall risk of damage to the optic nerve due to elevated IOP may be higher in this patient population.2,6,21,24 Ocular hypertension is reported to be found in 4 ~ 10% of the population over 40 yr of age,25 and the incidence of primary open-angle glaucoma is known to be increased by ten to 15 times in these patients.26 Patients with chronic open-angle glaucoma have reduced capacity for drainage of the aqueous humour; consequently, they have a reduced capacity to compensate for an acute rise in IOP. We found that 14/66 patients in this study actually had IOPs > 20 mmHg at baseline evaluation, and two patients were suspected as having normotensive glaucoma.
The decrease in OPP together with mechanical compression on ganglion cell axons is an important mechanism of optic neuropathy that is caused by increased IOP.2,21,27 The results of the present study have clinical significance given that propofol may decrease the risk of damage to the optic nerve during laparoscopic surgery by attenuating the increase in IOP and, therefore, decreasing the risk of ocular hypoperfusion.
The duration of pneumoperitoneum in the Trendelenburg position, ETCO2, and PIP are all known to contribute to increased IOP.2,7,28 One key determinant of IOP is the volume of the aqueous humour, which depends on the pressure gradient between IOP and episcleral venous pressure for drainage.29 Episcleral pressure increases when CVP increases, eventually causing IOP elevation. Although we found an increase in CVP in both groups following pneumoperitoneum and the Trendelenburg position, CVP was similar in both groups. Mechanical ventilation at high PIP is also known to cause an increase in IOP by increasing intrapulmonary pressure,28 and indeed, the relatively high PIP observed in our study seems to have contributed to the increase in IOP. On the other hand, while hyperventilation is known to decrease IOP,30 we were not able to find any significant relationship between ETCO2 and IOP in the present study. Overall, although the results of our study partially support those of previous reports, we were not able to identify any of the aforementioned factors as the cause of differences in IOP between the two groups as there were no intergroup differences in CVP, PIP, or ETCO2.
Adrenergic stimulation is known to increase IOP by increasing the resistance to the outflow of aqueous humour at the trabecular meshwork between the anterior chamber and Schlemm’s canal by causing vasoconstriction and venoconstriction.31 Patients usually experience high postoperative pain and anxiety, which leads to increased sympathetic tone during the acute postoperative period. This may explain the increased IOP at T6 in the Sevoflurane Group. Interestingly, this was not observed in the Propofol Group, a finding also reported by Sator et al.14 who measured increased IOPs after emergence from anesthesia with desflurane and isoflurane, but not after anesthesia with propofol. Since there is no guarantee that the depth of intraoperative anesthetic can be maintained at the level of MAC-BAR,32 it can be said that adrenergic stimulation exists to a variable degree throughout the operation. Based on the results of the present study, we can only assume that propofol-based TIVA may have a greater ability to alleviate adrenergic stimulation than sevoflurane and thus maintain IOP at a lower level. The underlying interaction between propofol and the activity of the autonomic nervous system during laparoscopic surgery in the steep Trendelenburg position remains to be studied.
This study has limitations. First, OPP was calculated from MAP and IOP, and blood flow was not measured at the optic nerve head. Second, the duration of surgery in this study was relatively short. Longer operation times have been shown to be correlated with ION in spine surgeries in the prone position33 as well as in surgeries performed in the steep Trendelenburg position.7 In this study, the mean duration of pneumoperitoneum in the Trendelenburg position was approximately 90 min; thus, the clinical effect of propofol-based TIVA to reduce IOP in patients undergoing procedures of a prolonged duration is not clear. Further studies conducted in longer robotic procedures are needed to explore this aspect. Lastly, several patients were found to present with extremely elevated IOP, which may not have been due simply to choice of anesthetic. While this may represent a confounding factor, exclusion of these patients would have been a possible cause of bias; therefore, patients with elevated IOP were included for analysis.
In conclusion, we found that propofol-based TIVA was more effective than inhalation anesthesia with sevoflurane in attenuating the increase in IOP during laparoscopic surgery requiring CO2 pneumoperitoneum in the steep Trendelenburg position. Although the primary outcome of this study was not the change in the actual incidence of postoperative ocular complications, IOP seems appropriate as a surrogate marker of ocular venous congestion and can therefore be considered to reflect the risk of ION. Overall, our results seem to have clinical significance given that this study was conducted in patients who are relatively vulnerable to the damaging effects of an elevation in IOP. The use of propofol-based TIVA may be of benefit in this patient population.
References
Gainsburg DM. Anesthetic concerns for robotic-assisted laparoscopic radical prostatectomy. Minerva Anestesiol 2012; 78: 596-604.
Molloy BL. Implications for postoperative visual loss: steep trendelenburg position and effects on intraocular pressure. AANA J 2011; 79: 115-21.
Goepfert CE, Ifune C, Tempelhoff R. Ischemic optic neuropathy: are we any further? Curr Opin Anaesthesiol 2010; 23: 582-7.
Weber ED, Colyer MH, Lesser RL, Subramanian PS. Posterior ischemic optic neuropathy after minimally invasive prostatectomy. J Neuroophthalmol 2007; 27: 285-7.
Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol 2008; 145: 604-10.
Pinkney TD, King AJ, Walter C, Wilson TR, Maxwell-Armstrong C, Acheson AG. Raised intraocular pressure (IOP) and perioperative visual loss in laparoscopic colorectal surgery: a catastrophe waiting to happen? A systematic review of evidence from other surgical specialities. Tech Coloproctol 2012; 16: 331-5.
Awad H, Santilli S, Ohr M, et al. The effects of steep trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg 2009; 109: 473-8.
Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology 2006; 105: 652-9.
Mowafi HA, Al-Ghamdi A, Rushood A. Intraocular pressure changes during laparoscopy in patients anesthetized with propofol total intravenous anesthesia versus isoflurane inhaled anesthesia. Anesth Analg 2003; 97: 471-4.
Grosso A, Scozzari G, Bert F, Mabilia MA, Siliquini R, Morino M. Intraocular pressure variation during colorectal laparoscopic surgery: standard pneumoperitoneum leads to reversible elevation in intraocular pressure. Surg Endosc 2013; 27: 3370-6.
Murphy DF. Anesthesia and intraocular pressure. Anesth Analg 1985; 64: 520-30.
Alexander R, Hill R. Lipham WJ, Weatherwax KJ, el-Moalem HE. Remifentanil prevents an increase in intraocular pressure after succinylcholine and tracheal intubation. Br J Anaesth 1998; 81: 606-7.
Schafer R, Klett J, Auffarth G, et al. Intraocular pressure more reduced during anesthesia with propofol than with sevoflurane: both combined with remifentanil. Acta Anaesthesiol Scand 2002; 46: 703-6.
Sator S, Wildling E, Schabernig C, Akramian J, Zulus E, Winkler M. Desflurane maintains intraocular pressure at an equivalent level to isoflurane and propofol during unstressed non-ophthalmic surgery. Br J Anaesth 1998; 80: 243-4.
Sator-Katzenschlager S, Deusch E, Dolezal S, et al. Sevoflurane and propofol decrease intraocular pressure equally during non-ophthalmic surgery and recovery. Br J Anaesth 2002; 89: 764-6.
Sugata A, Hayashi H, Kawaguchi M, Hasuwa K, Nomura Y, Furuya H. Changes in intraocular pressure during prone spine surgery under propofol and sevoflurane anesthesia. J Neurosurg Anesthesiol 2012; 24: 152-6.
Minto CF, Schnider TW, Shafer SL. Pharmacokinetics and pharmacodynamics of remifentanil. II. Model application. Anesthesiology 1997; 86: 24-33.
Coetzee JF, Glen JB, Wium CA, Boshoff L. Pharmacokinetic model selection for target controlled infusions of propofol. Assessment of three parameter sets. Anesthesiology 1995; 82: 1328-45.
Bathija R, Gupta N, Zangwill L, Weinreb RN. Changing definition of glaucoma. J Glaucoma 1998; 7: 165-9.
Kymes SM, Kass MA, Anderson DR, Miller JP, Gordon MO. Ocular Hypertension Treatment Study Group (OHTS). Management of ocular hypertension: a cost-effectiveness approach from the Ocular Hypertension Treatment Study. Am J Ophthalmol 2006; 141: 997-1008.
He Z, Vingrys AJ, Armitage JA, Bui BV. The role of blood pressure in glaucoma. Clin Exp Optom 2011; 94: 133-49.
Moore D, Harris A, Wudunn D, Kheradiya N, Siesky B. Dysfunctional regulation of ocular blood flow: a risk factor for glaucoma? Clin Ophthalmol 2008; 2: 849-61.
Butterworth JF, Mackey DC, Wasnick JD. Anesthesia for ophthalmic surgery. Morgan and Mikhail’s Clinical Anesthesiology, 5th ed. New York: McGraw-Hill Professional; 2013: 759-71
Awad H, Malik OS, Cloud AR, Weber PA. Robotic surgeries in patients with advanced glaucoma. Anesthesiology 2013; 119: 954.
Rao HL, Puttaiah NK, Babu JG, Maheshwari R, Senthil S, Garudadri CS. Agreement among 3 methods of optic disc diameter measurement. J Glaucoma 2010; 19: 650-4.
Leske MC, Connell AM, Wu SY, Hyman L, Schachat AP. Distribution of intraocular pressure. The Barbados Eye Study. Arch Ophthalmol 1997; 115: 1051-7.
Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol 2010; 4: 531-46.
Johnson DS, Crittenden DJ. Intraocular pressure and mechanical ventilation. Optom Vis Sci 1993; 70: 523-7.
Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J 2010; 4: 52-9.
Petounis AD, Chondreli S, Vadaluka-Sekioti A. Effect of hypercapnea and hyperventilation on human intraocular pressure general anaesthesia following acetazolamide administration. Br J Ophthalmol 1980; 64: 422-5.
Ismail SA, Bisher NA, Kandil HW, Mowafi HA, Atawia HA. Intraocular pressure and haemodynamic responses to insertion of the i-gel, laryngeal mask airway or endotracheal tube. Eur J Anaesthesiol 2011; 28: 443-8.
Gelb AW, Leslie K, Stanski DR, Shafer SL. Monitoring the Depth of Anesthesia. In: Miller RD (Ed.). Miller’s Anesthesia, 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010: 1229-65.
Lee LA, Newman NJ, Wagner TA, Dettori JR, Dettori NJ. Postoperative ischemic optic neuropathy. Spine (Phila Pa 1976) 2010; 35: S105-16
Funding
Funding was provided solely from departmental sources.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Young-Chul Yoo and Seokyung Shin contributed equally to this work. Young-Chul Yoo, Seokyung Shin, Eun Kyeong Choi, and Sun-Joon Bai contributed to the study design. Young-Chul Yoo, Seokyung Shin, Eun Kyeong Choi, Chan Yun Kim, and Young Deuk Choi contributed to the acquisition of data. Young-Chul Yoo, Seokyung Shin, Sun-Joon Bai contributed to the analysis of data. Young-Chul Yoo, Seokyung Shin, Eun Kyeong Choi, Chan Yun Kim, Young Deuk Choi, and Sun-Joon Bai helped prepare the manuscript.
Rights and permissions
About this article
Cite this article
Yoo, YC., Shin, S., Choi, E.K. et al. Increase in intraocular pressure is less with propofol than with sevoflurane during laparoscopic surgery in the steep Trendelenburg position. Can J Anesth/J Can Anesth 61, 322–329 (2014). https://doi.org/10.1007/s12630-014-0112-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0112-2