Abstract
Purpose
Cerebral desaturation occurs frequently in patients undergoing one-lung ventilation for thoracic surgery. The mechanism of this desaturation is unclear regarding its etiology. The objective of this study was to investigate whether or not decreases in cerebral oxygen saturation associated with one-lung ventilation were a consequence of decreased cardiac output.
Methods
A blinded observational study was conducted in 23 patients undergoing one-lung ventilation with thoracic surgery. Eighteen patients completed the study. Cerebral oxygen saturation was monitored using near-infrared spectroscopy (FORE-SIGHT® monitor). Invasive blood pressure was monitored and hemodynamic variables were interrogated using the FloTrac® system. Anesthesia was maintained with sevoflurane with a F i O2 of 1.0. Post-hoc analysis involved a comparison between baseline and integrated changes in cerebral saturation, heart rate, stroke index, cardiac index, and stroke volume variability.
Results
All patients showed cerebral desaturation from a baseline of two-lung ventilation in the lateral decubitus position following institution of one-lung ventilation. The cardiac index was stable at these times, but with one-lung ventilation, the heart rate decreased and the stroke index increased to maintain a stable product. The integral of heart rate × time was inversely correlated with the integral of cerebral desaturation × time (linear regression analysis; P = 0.02; (df) = 16)).
Conclusions
Cerebral oxygen desaturation was universal during one-lung ventilation in this study. There was no correlation between cerebral desaturation and cardiac output or other hemodynamic variables.
Résumé
Objectif
La désaturation cérébrale survient fréquemment chez les patients subissant une ventilation sélective pendant une chirurgie thoracique. L’étiologie du mécanisme de cette désaturation n’est pas claire. L’objectif de cette étude était d’évaluer si les réductions de la saturation cérébrale en oxygène associées à une ventilation sélective étaient - ou non - une conséquence d’un débit cardiaque réduit.
Méthode
Une étude observationnelle en aveugle a été réalisée auprès de 23 patients subissant une ventilation sélective lors d’une chirurgie thoracique. Dix-huit patients ont complété l’étude. La saturation cérébrale en oxygène a été monitorée à l’aide de spectroscopie proche infrarouge (moniteur FORE-SIGHT®). La tension artérielle a été monitorée de façon effractive et les variables hémodynamiques ont été interrogées à l’aide du system FloTrac®. L’anesthésie a été maintenue à l’aide de sévoflurane avec une F i O2 de 1,0. L’analyse post-hoc comprenait une comparaison entre les valeurs de base et les changements intégrés de la saturation cérébrale, la fréquence cardiaque, l’index systolique, l’index cardiaque, et la variabilité du volume d’éjection.
Résultats
Tous les patients ont manifesté une désaturation cérébrale après la mise en place de la ventilation sélective par rapport au moment précédant l’intervention, où ils se trouvaient en ventilation bipulmonaire et en position de décubitus latéral. L’index cardiaque était stable à ces moments-là, mais pendant la ventilation sélective, la fréquence cardiaque a diminué et l’index systolique augmenté afin de maintenir un produit stable. L’intégrale de la fréquence cardiaque × le temps était inversement corrélée à l’intégrale de la désaturation cérébrale × le temps (analyse de régression linéaire; P = 0,02; (degrés de liberté) = 16)).
Conclusion
La désaturation cérébrale en oxygène était systématique pendant la ventilation sélective dans cette étude. Aucune corrélation n’a été observée entre la désaturation cérébrale et le débit cardiaque ou d’autres variables hémodynamiques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Monitoring of cerebral oxygen saturation by near-infrared spectroscopy (NIRS) is being used more frequently to aid perioperative management of complex anesthesia and surgery.1,2 Reduced perioperative cerebral saturation correlates with greater morbidity and mortality in patients undergoing open heart surgery3 and major abdominal procedures4 and also in patients following thoracic surgery.5 Low cerebral saturation correlates with postoperative delirium and postoperative cognitive deficit.6
Thoracotomy is reported to be associated with a very high incidence of cerebral desaturation when one-lung ventilation (OLV) is instituted.5,7 The mechanism of cerebral desaturation in this context is unclear because, in the majority of cases, pulse oximetry indicates that arterial oxygen saturation remains well within clinically acceptable levels.
The possible causes of cerebral desaturation during thoracic surgery include increases in cerebral oxygen utilization, unlikely in the presence of general anesthesia, or a reduction in cerebral oxygen delivery that could be due to a reduction either in arterial oxygen content or in cerebral blood flow that might also be related to a decrease in cardiac output. Understanding the cerebral hemodynamic consequences following institution of OLV could aid in differentiating these causes. The physiologic consequences of superimposing OLV on the lateral decubitus position required for surgery include increases in pulmonary vascular resistance and pulmonary arteriovenous shunt, hypoxic pulmonary vasoconstriction, and the activation of inflammatory pathways that may culminate in a reduction in alveolar and arterial oxygen tension.8 With the increase in pulmonary resistance during collapse of the whole lung, right ventricular (and subsequently left ventricular) cardiac output would be expected to decrease, especially under anesthesia where compensatory reflex mechanisms may be blunted. Furthermore, with impaired right ventricular performance, an increase in right-sided filling pressures could increase cerebral venous blood volume and affect cerebral saturation in this manner. Thus, in susceptible patients, these mechanisms could contribute to the decreases seen in cerebral oxygen saturation.
Recently, less invasive cardiac output monitors have been interfaced with an indwelling arterial cannula (a standard hemodynamic monitor during thoracic surgery) to offer a means to facilitate continuous measurement of cardiac output.9 One of these monitors, the FloTrac® system, calculates the variation of cardiac output and stroke volume in real time.
Organs rely on both blood pressure and flow for optimal function. Continuous and simultaneous measurements of both cardiac output and cerebral oxygen saturation (ASctO2) may provide insight into the nature of the cerebral desaturation seen with OLV. Accordingly, we hypothesized that cerebral desaturation would be a result of a reduction in cardiac output that could then compromise oxygen flow to the brain during OLV.
Methods
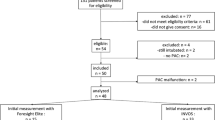
The Biomedical Research Ethics Board at the University of Manitoba approved this prospective observational study, and written informed consent was obtained from all participants prior to surgery. From September 2010 to December 2010, we recruited patients presenting for elective video-assisted thoracoscopic surgery or open thoracotomy involving OLV with an anticipated duration longer than 45 min. Twenty-three American Society of Anesthesiologists physical class I-III patients enrolled in the study. Exclusion criteria included patients weighing more than 120 kg, those with significant aortic regurgitation, or those with any cutaneous condition or otherwise that interfered with the application of the cerebral oximeter sensors.
A thoracic epidural catheter was inserted in patients undergoing an open thoracotomy so that a continuous infusion of 0.25% bupivacaine at 4-8 mL·hr−1 could be administered throughout the operative procedure. Standard monitors were applied, including a five-lead electrocardiogram and a noninvasive blood pressure cuff, and peripheral oxygen saturation was measured using pulse oximetry. A radial arterial cannula was placed, and an arterial blood gas sample was then drawn with the patient breathing room air. Monitoring with a FORE-SIGHT® (CasMed, Branford, CT, USA) cerebral oximeter was undertaken via bilateral sensors applied to the patient’s forehead. Prior to induction, cerebral saturation was noted with the patient breathing room air. The FloTrac system (Edwards Life Sciences, Irvine, CA, USA), which utilizes a proprietary algorithm to calculate stroke volume based on the arterial pulse contour, was used to measure cardiac output.
All patients received a standardized anesthetic, including pre-medication with midazolam 1-2 mg iv. After pre-oxygenation, anesthesia was induced with fentanyl 2-4 μg·kg−1 or sufentanil 0.2-0.4 μg·kg−1, propofol 1-2 mg·kg−1, and rocuronium 0.6 mg·kg−1. A left-sided double-lumen endotracheal tube was then placed under fibreoptic bronchoscope guidance, and the patient was then placed in the lateral decubitus position with the non-operative side dependent. Anesthesia was maintained with sevoflurane (0.5-1.0 MAC) on a F i O2 of 1.0 for the duration of the procedure. Additional narcotics and muscle relaxants were administered at the discretion of the attending anesthesiologist. The baseline measurement of the average ASctO2 was considered the highest cerebral saturation on 100% oxygen. These measurements were taken after induction of anesthesia and lateral decubitus positioning, during the period of two-lung ventilation, and within ten minutes of commencing OLV. The anesthesiologist was blinded to ASctO2 and cardiac output values. Blood gas analysis was performed at 15-min intervals to a maximum of two hours. The following data were recorded in one-second intervals using Trendface Solo® (iExcellence, Germany) software on a laptop personal computer: blood pressure, heart rate, calculated cardiac output, calculated cardiac index, stroke volume, calculated heart rate (calculated heart rate = calculated cardiac output/stroke volume), stroke index, stroke volume variability, and ASctO2.
Patients’ lungs were ventilated with a tidal volume of 8 mL·kg−1 ideal body weight and then lowered to 4 mL·kg−1 with initiation of OLV. Respiratory frequency was titrated to maintain a PaCO2 < 50 mmHg. If peripheral saturations decreased to < 90%, the anesthesiologist intervened to improve oxygenation, including the use of a positive end-expiratory pressure of 5 cm H2O.
Cerebral oxygen saturation (left-sided, right-sided, and average) was measured continuously throughout the case. A decrease from baseline during two-lung ventilation to a cerebral saturation ≤ 55% was deemed to be a major cerebral desaturation. If such events persisted for more than five minutes, they were communicated to the attending anesthesiologist to allow for intervention.
Data were analyzed post hoc based on changes in ASctO2 derived from the second-by-second output from the data acquisition system. Changes in ASctO2 were indexed to the baseline measurement. The nadir of ASctO2 with OLV was determined and established the maximal delta ASctO2 for that patient. Irrespective of the maximal difference, the integrated decrease in ASctO2 was calculated second-by-second from baseline for the duration of OLV. This integrated decrease for cerebral desaturation was correlated with similarly derived integrated changes in heart rate, stroke index, and cardiac index referenced to the same time period as for maximal ASctO2. The integrated changes were normalized to the patient with the shortest period of OLV, i.e., 57 min (duration of OLV varied from 57-159 min). The normalized integrated changes for heart rate and cardiac index were also compared. These analyses were undertaken with a specially written program in LabVIEW (National Instruments Canada, Vaudreuil QC, Canada).
Statistical analysis
Mixed effects repeated measures models were used to assess whether significant time trends existed. Post-hoc comparisons were adjusted for multiplicity via a simulation approach; the simulation adjustment computes adjusted P values and confidence limits. SAS® version 9.2 (SAS Institute, Cary NC, USA) was used for all analyses. Linear regression analysis was undertaken to compare various integrated indices. P values < 0.05 were considered significant.
Results
Twenty-three patients were enrolled in the study, and data were analyzed on 18 patients. Four patients were excluded due to protocol violations (three due to the standardized anesthetic and one due to the duration of OLV being < 45 min). Patient demographics and surgical procedure are summarized in the Table.
The effect of time on hemodynamic variables is shown in Fig. 2. Heart rate decreased with initiation of anesthesia and further decreased with institution of OLV. Mean arterial pressure was maximal prior to induction of anesthesia, but not statistically significant, and it was essentially stable over the course of the procedure. Stroke index decreased post induction but then increased again with the onset of OLV. Cardiac index did not differ when comparing the period of two-lung ventilation with that of OLV. Higher values were seen prior to induction of anesthesia and following reinstitution of two-lung ventilation. Stroke volume variability decreased by approximately one-half with the institution of OLV and remained diminished with acute re-inflation of the lungs.
Prior to induction of anesthesia, the ASctO 2 (Standard Deviation) as 72 ± 5% while breathing room air. This increased to 85 ± 5% after induction with a F i O2 of 1.0 during two-lung ventilation. The nadir ASctO 2 during OLV was 72 ± 5%. This nadir was not significantly different from the awake room air saturation; however, it was a mean reduction of 13% from the highest ASctO2 during two-lung ventilation in the lateral decubitus position determined before commencing OLV (Figure 3). All 18 patients showed a decrease in ASctO2 from the defined baseline. In four patients, the decrease in saturation was ≤ 5%. In the other 14 patients, the decrease was > 10% with a maximal decrease of 27% seen in one patient. Five patients had a decrease in saturation of ≥ 20%. The lowest saturation was 62%, and there were five patients with saturations of ≤ 65%.
Post induction, with a F i O2 of 1.0, the mean peripheral saturation was 99.6 (1)%. The mean peripheral saturation at oxygen saturation (SctO2) nadir was 98 (4)%. The single patient with a pulse oximetry desaturation to 94% was also the patient with the lowest baseline peripheral saturation. The arterial oxygen tension (PaO2) was 83 (10) mmHg prior to induction of anesthesia. It increased to 397 ± 134 mmHg post induction with a F i O2 of 1.0, with a nadir on OLV of 143 ± 62 mmHg. When patients had ventilation restored to two lungs with a F i O2 of 1.0, the PaO2 was 358 ± 114 mmHg, which was not significantly different from prior to OLV . The PaCO2 increased throughout the procedure from 36 ± 3 mmHg pre-induction to 46 ± 4 mmHg post induction on two-lung ventilation. During the period of OLV, carbon dioxide tension was essentially unchanged from that seen with both lungs ventilated, i.e., 47 ± 6 mmHg.
The normalized integral of % change × time of decrease in ASctO2 following OLV was 26,731 ± 15,858 %·sec−1 (range 796-56,974) (all decreases were measured from baseline). The normalized integral in the same time period for heart rate was -34,447 ± 36,922 beats·sec−1 (range -118,269 to 15,810), a negative value representing a decrease in heart rate from baseline. The normalized integral in the same time period for stroke index was 244,833 ± 65,877 mL·m−2·sec−1 (range 164,398-467,529), a positive value representing an increase in stroke index from baseline. Correlations between the various computed integrated changes are shown in Fig. 1. A significant inverse correlation was seen between the integral for change in ASctO2 and the integral for change in heart rate (Fig. 1A). There was no significant correlation seen between the integrals relating changes in ASctO2 and stroke index (Fig. 1B). As well, there was no significant correlation seen between the integrals of changes in ASctO2 and calculated cardiac index (Fig. 1C).
A comparison of normalized integrated changes. A) Heart rate vs ASctO2; an inverse correlation was present; P value for the linear regression analysis = 0.020. B) Stroke index vs ASctO2; no correlation existed. C) Calculated cardiac index vs ASctO2; no correlation existed. ASctO2 = cerebral oxygen saturation; delta ASctO2 = ∆ASctO2
Discussion
Our findings confirm prior work indicating that cerebral desaturation occurs with the onset of OLV during thoracic surgery. We observed a decrease in cerebral oxygen saturation in all patients, whether they were undergoing open procedures or video-assisted thoracotomy. Given the recommended implementation of lower inspired concentrations of oxygen during thoracic surgery,12 this recommendation needs to be weighed against the evidence of cerebral desaturation with a F i O2 of 1.0 during OLV, as described in this study and in prior work.
Our finding of a universal cerebral oxygen desaturation with the onset of OLV may be due in part to our choice of baseline, which was the maximal cerebral saturation on two-lung ventilation in the lateral decubitus position within ten minutes prior to lung collapse. We chose this baseline for several reasons. First, it represents a baseline comparison while the patient remains in the same position under stable anesthetic conditions and on the same inspired concentration of oxygen; therefore it permits an accurate assessment of the single intervention of lung collapse. In addition, prior work has indicated that meaningful alterations in cerebral saturation can be determined in this manner, for example, pre- and post-carotid cross-clamping with carotid endarterectomy.10,11 Lastly, establishing an a priori agreed upon baseline permits true comparisons across the maximal number of studies.7
In these experiments, we show desaturations using the same type of cerebral oximeter (i.e., CasMed FORE-SIGHT) used by Hemmerling et al.7 This oximeter claims absolute cerebral oximetry, not just documentation of trends. The magnitude of the mean decrease in cerebral saturation from baseline in this study is similar to that which correlated to the increased morbidity seen with carotid cross-clamping.10 While not equivalent to an ipsilateral occlusion of blood flow to a cerebral hemisphere, the magnitude of the cerebral desaturation suggests that it is important to have a better understanding of the problem.
Also, similar to the work by Hemmerling et al.,7 we observed a worrisome disconnect between peripheral saturation and cerebral saturation. This observation is of particular concern as pulse oximetry is a standard monitor used as a surrogate for tissue and cerebral oxygenation. Indeed, the majority of the cerebral desaturations occurred with arterial pulse oximetry readings of ≥ 98% while on a F i O2 of 1.0. We point out that current management of OLV calls for a titration to lower F i O2 values as long as peripheral saturation is acceptable.12 Such a strategy raises the potential concern that cerebral hypoxia may be initiated or exacerbated with this approach.
The continuous measurement of cardiac output and its index showed an interesting association with OLV. While mean cardiac index was unaltered when transitioning from two-lung to OLV, the heart rate × stroke index changed inversely to maintain the product relatively constant, that is, heart rate decreased and stroke index increased by approximately similar magnitudes. The uniformity of changes for the integral of heart rate response during OLV (with 14/18 patients with a negative integral from baseline) as well as for the integral of stroke index (with 18/18 patients with a positive integral from baseline), suggests the presence of a reflex mechanism in response to lung collapse. Our observations imply that the potential for cerebral ischemia with OLV is indeed present and manifests incrementally as shown with the inverse correlation between changes over time in heart rate response and the magnitude of cerebral desaturation (Fig. 1A). No such correlations were seen with integral changes in stroke index or cardiac index for the integral changes in cerebral saturation (Figs. 1B and 1C). There was a non-significant relationship between an increased integral of cardiac index over time with the integral of cerebral desaturation over time (P = 0.24). The study is underpowered if such a relationship is present. If the R2 value of the correlation were unaltered, 47 patients would need be studied for this relationship to be significant at the P = 0.05 level. Our initial hypothesis was the converse, i.e., that cerebral desaturation could be a consequence of decreased cardiac output. Either way, the possibility of a beta error based on small sample size must be a consideration.
Recent work by Suehiro and Okutai13 suggests that magnitude and duration of cerebral desaturation seen with OLV may have clinical relevance. Their work showed a significant decrease from baseline in mini-mental state examination scores in those patients with longer periods of desaturation. Kazan et al.5 also showed cognitive problems with a decrease in cerebral oxygen saturation as measured from a baseline post induction on a F i O2 of 1.0. Further, an intraoperative decrease in cerebral saturation of ≥ 12% with cross-clamping during carotid endarterectomy under general anesthesia is associated with increased patient morbidity.10
This study combined two fairly recent approaches designed to provide continuous data output for patient monitoring, i.e., cerebral oximetry and cardiac output. A number of competing technologies exist. Our findings confirm those of Hemmerling et al. using the FORE-SIGHT cerebral oximeter in both studies.7 We have also used this monitor to document changes in cerebral saturation with cross-clamping during carotid endarterectomy under general anesthesia.11 Cardiac output measurement utilizing the FloTrac system has been recently reviewed.14 This device has been found to provide a good estimate of cardiac output compared with pulmonary artery thermodilution, which is considered the gold standard; however, other work highlights the potential limitation of the currently available minimally invasive cardiac output devices when compared with thermodilution.15 The ability to monitor cardiac output in a minimally invasive manner was beneficial for this study.
One limitation of this study and others currently in the literature is a critical lack of an index of cerebral blood flow. Given our finding of well-oxygenated arterial blood during the period of OLV, one mechanism our observations suggest is that cerebral desaturation results from an altered supply/demand ratio. This would mean that cerebral blood flow is decreased more that the metabolic demand, which is depressed with general anesthesia. An intraoperative measure of cerebral flow or its surrogate, cerebral blood flow velocity, by Doppler ultrasonography is necessary to provide further insight. Also of importance, especially if reflex mechanisms are involved, is a quiescent period without surgical intervention for careful study of the transition from two-lung to OLV. Only then, with the caveats of a stable depth of anesthesia and stable end-tidal oxygen and carbon dioxide tensions, can the mechanisms and hemodynamic responses responsible for cerebral desaturation be delineated. As stated above, an agreed upon baseline to allow comparison and contrast between studies is also necessary. The choice of a baseline is critically important because the magnitude of the various integrated changes varies dramatically depending on the choice. It may well be that a full study of the mechanisms involved may require an animal model for rigid control of the experimental paradigm.
With respect to the desaturations that occur during OLV, other causative explanations are possible. For example, there is the potential of central venous pressure increases during OLV, which could result in increased back pressure for the cerebral venous circulation, a possibility yet to be examined. The cerebral oximeter employs an algorithm that assumes a 70% contribution from the venous blood such that an increased cerebral venous blood volume could potentially account for the desaturation signal seen. Nonetheless, in their study in dogs, Cohen et al. 16 did not find an increase in central venous pressure with OLV. More recent work indicates an inverse correlation between stroke volume variability and right ventricular diastolic volume index.17 We observed a marked decrease in stroke volume variation with the onset of OLV, implying a fuller pulmonary vasculature, perhaps associated with increased venous pressure.
Additionally, there would be merit in studying the incidence of cerebral desaturation with decreases in F i O2, which have been proposed and clinically implemented with the goal of lung protection. Given the lack of correlation between peripheral and cerebral saturation, perhaps increased cerebral desaturation with lower F i O2 would go undetected and put patients at further risk of non-pulmonary complications.
In summary, we confirm that OLV is associated with cerebral oxygen desaturation. We report that the magnitude of cerebral desaturation is correlated with increasing bradycardia. Given the lack of correlation between peripheral oxygen saturation and cerebral oxygen saturation shown in this and other studies, we suggest that it may be prudent to monitor cerebral oxygenation for protection of the brain when anesthetizing patients in cases where lung protection strategies utilizing F i O2 limitation are deemed important.
References
Murkin JM. NIRS: a standard of care for CPB vs. an evolving standard for selective cerebral perfusion? J Extra Corpor Technol 2009; 41: 11-4.
Murkin JM. Applied neuromonitoring and improving central nervous system outcomes. Artif Organs 2008; 32: 851-5.
Slater JP, Guarino T, Stack J, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg 2009; 87: 36-44; discussion 44-5.
Casati A, Fanelli G, Pietropaoli P, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg 2005; 101: 740-7.
Kazan R, Bracco D, Hemmerling TM. Reduced cerebral oxygen saturation measured by absolute cerebral oximetry during thoracic surgery correlates with postoperative complications. Br J Anaesth 2009; 103: 811-6.
Schoen J, Meyerrose J, Paarmann H, Heringlake M, Hueppe M, Berger KU. Preoperative regional cerebral oxygen saturation is a predictor of postoperative delirium in on-pump cardiac surgery patients: a prospective observational trial. Crit Care 2011; 15: R218.
Hemmerling TM, Bluteau MC, Kazan R, Bracco D. Significant decrease of cerebral oxygen saturation during single-lung ventilation measured using absolute oximetry. Br J Anaesth 2008; 101: 870-5.
Wright CD, Gaissert HA, Grab JD, O’Brien SM, Peterson ED, Allen MS. Predictors of prolonged length of stay after lobectomy for lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk-adjustment model. Ann Thorac Surg 2008; 85: 1857-65; discussion 1865.
Funk DJ, Moretti EW, Gan TJ. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg 2009; 108: 887-97.
Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 2009; 103(Suppl 1): i3-13.
Espenell AE, McIntyre IW, Gulati H, et al. Lactate flux during carotid endarterectomy under general anesthesia: correlation with various point-of-care monitors. Can J Anesth 2010; 57: 903-12.
Grocott HP. Oxygen toxicity during one-lung ventilation: is it time to re-evaluate our practice? Anesth Clin 2008; 26: 273-80.
Suehiro K, Okutai R. Duration of cerebral desaturation time during single-lung ventilation correlates with mini mental state examination score. J Anesth 2011; 25: 345-9.
Mayer J, Boldt J, Poland R, Peterson A, Manecke GR Jr. Continuous arterial pressure waveform-based cardiac output using the FloTrac/Vigileo: a review and meta-analysis. J Cardiothorac Vasc Anesth 2009; 23: 401-6.
Peyton PJ, Chong SW. Minimally invasive measurement of cardiac output during surgery and critical care: a meta-analysis of accuracy and precision. Anesthesiology 2010; 113: 1220-35.
Cohen, E, Eisenkraft, JB, Thys, DM, Kirschner, PA Kaplan J. Oxygenation and hemodynamic changes during one-lung ventilation: effects of CPAP10, PEEP10 and CPAP10/PEEP10. J Cardiothorac Anesth 1988; 2: 34-40.
Su BC, Tsai YF, Cheng CW, et al. Stroke volume variation derived by arterial pulse contour analysis is a good indicator for preload estimation during liver transplantation. Transplant Proc 2012; 44: 429-32.
Acknowledgements
We sincerely thank Dr. H. Unruh, Dr. L. Tan, and Dr. S. Srinathan from the Department of Thoracic Surgery at the University of Manitoba for their assistance in this project. The senior author (W.A.C.M.) thanks Dr. James Duffin, Professor Emeritus of Physiology and Anesthesia, University of Toronto, for his instruction on the LabVIEW program used for data analysis while he was on sabbatical in Toronto.
Funding
Funding provided through unrestricted grants from the Winnipeg Health Sciences Centre Research Foundation and the Academic Oversight Committee of the Department of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is accompanied by an editorial. Please see Can J Anesth 2013; 60: this issue.
Author contributions
Ryan Brinkman, resident researcher, was involved and made substantial contributions to study conception, acquisition of data, and drafting the article. Ryan Brinkman and Duane Funk, investigator, made important contributions to study design, data interpretation, and manuscript revision Ryan Amadeo, principal investigator, and W. Alan C. Mutch, senior investigator, were involved in in all aspects of project design, data analysis, and interpretation, and article preparation and revision. Linda Girling, research associate, and Hilary Grocott, investigator, were involved in study design, data interpretation and analysis, and manuscript preparation.
Rights and permissions
About this article
Cite this article
Brinkman, R., Amadeo, R.J.J., Funk, D.J. et al. Cerebral oxygen desaturation during one-lung ventilation: correlation with hemodynamic variables. Can J Anesth/J Can Anesth 60, 660–666 (2013). https://doi.org/10.1007/s12630-013-9954-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-013-9954-2