Abstract
Purpose
Intravenous lidocaine given both intraoperatively and postoperatively decreases pain scores, reduces opioid consumption, and promotes faster return of bowel function following abdominal surgery. The purpose of this trial was to determine if intravenous lidocaine limited to the intraoperative period reduces length of hospital stay and improves functional recovery following abdominal hysterectomy.
Methods
Following Research Ethics Board approval and informed consent, women of American Society of Anesthesiologists’ class I and II undergoing abdominal hysterectomy were assigned randomly to lidocaine and control groups. Lidocaine subjects received an intravenous bolus of 1.5 mg·kg−1 followed by an infusion of 3 mg·kg−1·hr−1, while control subjects received matching placebo. Patients, anesthesiologists, and study personnel were blinded, and anesthesia and multimodal perioperative analgesia were standardized. The primary outcome of this trial was discharge from hospital on or before the second postoperative day (POD2). Additional criteria were assessed for secondary outcomes, i.e., discharge fitness on POD2, length of hospital stay, opioid use, numeric rating scores for pain, quality of recovery, and recovery of bowel function.
Results
Ninety of the 93 women who were recruited completed the study protocol. The characteristics of the patients in both groups were similar—lidocaine group (n = 44) and control group (n = 46)—and no difference was noted between groups in the numbers of women discharged from hospital on POD2 (10 lidocaine, 15 control; P = 0.295). Days to discharge fitness (P = 0.666) and length of hospital stay (P = 0.456) were also similar. Differences in opioid consumption, pain scores, and recovery were neither clinically nor statistically significant.
Conclusion
Intraoperative administration of intravenous lidocaine did not reduce hospital stay or improve objective measures of analgesia and recovery following abdominal hysterectomy. This trial was registered at ClinicalTrials.gov (NCT00382499).
Résumé
Objectif
La lidocaïne intraveineuse administrée pendant et après l’opération réduit les scores de douleur, diminue la consommation d’opioïdes et permet un retour plus rapide à une fonction intestinale normale après une chirurgie abdominale. L’objectif de cette étude était de déterminer si la lidocaïne intraveineuse, administrée exclusivement en période peropératoire, réduisait la durée de séjour à l’hôpital et améliorait la récupération fonctionnelle après une hystérectomie abdominale.
Méthode
Après avoir reçu l’autorisation du Comité d’éthique de la recherche et obtenu le consentement éclairé des patientes, des patientes de classe ASA (American Society of Anesthesiologists) I et II subissant une hystérectomie abdominale ont été randomisées en deux groupes, soit lidocaïne et témoin. Les patientes allouées au groupe lidocaïne ont reçu un bolus intraveineux de 1,5 mg·kg−1 suivi d’une perfusion de 3 mg·kg−1, alors que les patientes du groupe témoin ont reçu un placebo équivalent. Les patientes, les anesthésiologistes et le personnel de l’étude étaient tous en aveugle, et l’anesthésie et l’analgésie périopératoire multimodale étaient standardisées. Le critère de recherche principal de cette étude était le congé de l’hôpital au deuxième jour postopératoire (J2) ou avant. D’autres critères ont également été évalués pour mesurer des résultats secondaires, soit l’aptitude à recevoir le congé à J2, la consommation d’opioïdes, les scores sur les échelles numériques de douleur, la qualité de la récupération et la récupération d’une fonction intestinale normale.
Résultats
Quatre-vingt-dix des 93 patientes recrutées pour l’étude ont terminé le protocole. Les caractéristiques des patientes étaient semblables dans les deux groupes—groupe lidocaïne (n = 44) et groupe témoin (n = 46)—et aucune différence n’a été observée entre les groupes quant au nombre de femmes recevant leur congé de l’hôpital à J2 (10 lidocaïne, 15 témoin; P = 0,295). Les jours jusqu’à l’aptitude à recevoir son congé (P = 0,666) et la durée de séjour hospitalier (P = 0,456) étaient également semblables. Les différences de consommation d’opioïdes, de scores de douleur et de récupération n’étaient pas significatives d’un point de vue clinique ou statistique.
Conclusion
L’administration peropératoire de lidocaïne intraveineuse n’a pas réduit la durée de séjour à l’hôpital ni amélioré les mesures objectives de l’analgésie et de la récupération après une hystérectomie abdominale. Cette étude est enregistrée sous ClinicalTrials.gov (NCT00382499).
Similar content being viewed by others
Abdominal hysterectomy is the most common surgical procedure performed on women over the age of 35. Over one-fifth of Canadian women questioned in the 1998/99 National Population Health Survey reported having undergone a hysterectomy.Footnote 1 Since this procedure is so prevalent, perioperative care plans to reduce the length of hospital stay following hysterectomy could have a significant impact on the use of hospital resources. Opioid-sparing analgesic techniques are associated with achieving earlier recovery milestones, such as resumption of oral feeding and ambulation as well as reductions in length of hospital stay.1 Oral analgesics like non-steroidal anti-inflammatory drugs (NSAIDs) and gabapentinoids improve analgesia following hysterectomy,2 but they may be difficult to administer should oral intake be limited by ileus or postoperative nausea and vomiting (PONV). Systematic review of the literature identified that intravenous infusions of lidocaine during abdominal surgery were associated with decreases in duration of ileus, PONV, numeric rating scores (NRS) for pain, and length of hospital stay.2 It should be noted, however, that decreases in length of stay were less than one day (weighted mean difference -0.84 days, 95% confidence interval -1.38 to -0.31) and were associated with considerable heterogeneity (I 2 = 46.7%). Similarly, all studies infused lidocaine during surgery, but the duration of postoperative therapy was variable, ranging from zero to 24 hr.2 A treatment limited to the intraoperative period that demonstrates a consistent reduction in length of stay by a day or more would be adopted easily and would likely change institutional models of care.
Prior to commencing the present study, we documented that 21% of patients undergoing abdominal hysterectomy in our institution, a 1,000 bed academic tertiary care centre, were discharged on the second postoperative day (POD2). Our goal was to increase the proportion of POD2 discharges to 50% in order to convince our surgical colleagues to re-evaluate their current models of care. The purpose of the present study was to evaluate the influence of intraoperative infusion of lidocaine (1.5 mg·kg−1 bolus followed by an infusion of 3 mg·kg−1·hr−1 until the end of surgery) on the proportion of patients discharged on POD2 following abdominal hysterectomy. Our hypothesis was that 50% of subjects allocated to the lidocaine treatment group would be discharged on POD2 and improvement in POD2 discharge rates would be associated with more rapid attainment of discharge fitness, improved analgesia, and fewer side effects.
Methods
This randomized, blinded placebo-controlled trial follows the CONSORT statement for reporting the results of randomized trials.3 Following Research Ethics Board approval at the Ottawa Hospital, women ages 30-69 yr undergoing abdominal hysterectomy (with or without oopherectomy) were assessed for study eligibility. Pre-admission unit nurses identified eligible patients at the time of their preoperative evaluation and notified research personnel who then approached the patients regarding participation in the study. The following information was documented on all patients assessed: age, sex, height, weight, body mass index, serum creatinine, calculated creatinine clearance (Cockroft-Gault), primary diagnosis, scheduled procedure, and all medications. Patients with the following conditions were excluded: 1) American Society of Anesthesiologists’ physical status class III, IV, and V; 2) body mass index < 18.5 or > 30 kg·m−2; 3) unable to use patient-controlled analgesia; 4) history of liver dysfunction; 5) creatinine clearance < 50 mL·min−1, as calculated by the Cockroft-Gault formula; 6) history of seizure disorder; 7) hypersensitivity or allergy to amide-type local anesthetics study medications; 8) chronic pain syndromes; and 9) opioid use more than once per week. Analgesia and pain histories were elicited at the preoperative visit using the Brief Pain Inventory (BPI). Written informed consent was obtained prior to the day of surgery.
Consenting patients were assigned to lidocaine and control groups using randomization schedules prepared by a research manager not involved with the bedside care of patients in the study. The randomization schedule was derived from a computer-generated random numbers table. Separate randomization schedules were stratified by hospital campus (Civic and General Campuses); subjects within each schedule were blocked in groups of four. Campus-specific randomization schedules were held by the research pharmacist at each campus. Several hours before surgery, research personnel contacted the campus pharmacist who assigned the patient a unique study ID number according to the campus-specific randomization schedule. Study medications for lidocaine and control groups were then prepared by the pharmacist in identical syringes labelled only with the patient’s unique study number. Research personnel, patients, and attending anesthesiologists were blinded to the contents of the syringes. Two syringes were provided: a) a 10-mL syringe containing either 2% lidocaine or 0.9% saline for the loading dose and b) a 60-mL syringe containing either 2% lidocaine or 0.9% saline for the maintenance infusion, respectively.
All patients received a standardized balanced general anesthetic. Both the patients and the attending anesthesiologists remained unaware of group allocation throughout the study. One hour before surgery, the patients were premedicated with celecoxib 400 mg and acetaminophen 975 mg. An intravenous infusion of lactated Ringer’s solution was started in the operating room. Total fluid intake was restricted to 20-40 mL·kg−1. Prior to induction of anesthesia, patients in the lidocaine and control groups received a 0.075 mL·kg−1 loading dose of study medication (equivalent to 1.5 mg·kg−1 in the lidocaine group). Anesthesia was then induced with midazolam 20 μg·kg−1, fentanyl 3 μg·kg−1, and propofol titrated to loss of lid-lash reflex. Use of muscle relaxants was at the discretion of the attending anesthesiologist. Following induction, a continuous infusion of study medication was started at a rate of 0.15 mL·kg−1·hr−1 (equivalent to 3 mg·kg−1·hr−1 in the lidocaine group). Anesthesia was maintained with continuous infusion of fentanyl 2 μg·kg−1·hr−1 and halogenated anesthetic (either sevoflurane or desflurane) in air:oxygen at the discretion of the attending anesthesiologist. Halogenated anesthestics were adjusted using M-BIS (Aspect Medical Systems, Norwood, MA, USA) monitoring to maintain a M-BIS reading of 40-55. All patients received antiemetic prophylaxis with dexamethasone 8 mg and ondansetron 4 mg. All wounds were infiltrated with 20 mL of 0.25% bupivacaine with adrenaline at skin closure.
Postoperatively, all patients received celecoxib 200 mg po q12hr and acetaminophen 650 mg po q4hr until hospital discharge. Intravenous patient-controlled morphine was prescribed with the following settings: boluses of 0.02 mg·kg−1, no continuous infusion, and a one-hour maximum of 0.16 mg·kg−1·hr−1. Intravenous analgesia was discontinued when the patient tolerated a clear fluid diet. Morphine 5-10 mg po q4hr prn was ordered for pain that was not controlled with celecoxib and acetaminophen. Analgesia following discharge from hospital was at the discretion of the attending gynecologist.
Outcome measures were recorded by study personnel blinded to treatment allocation. Study personnel documented the day and time of discharge fitness and notified the surgical service when discharge criteria were fulfilled. The primary outcome, i.e., the proportion of patients discharged on POD2, was defined by the date the patient left hospital. Secondary outcomes were defined as follows: Discharge fitness was determined when the patient fulfilled all criteria in our institutional abdominal hysterectomy care pathway. Discharge criteria included: a) pain controlled with oral analgesics; b) stable vital signs; c) afebrile; d) passing flatus; e) scant or no vaginal discharge; f) incision clean, dry, and intact; g) full diet tolerated; and h) understands discharge instructions regarding staple removal, analgesia, and follow-up. Pain was assessed at rest and with coughing using an 11-point verbal NRS anchored at 0 for no pain and 10 for worst pain imaginable. Narcotic consumption was documented from patient-controlled analgesia pumps and the nursing record. All narcotic use was converted to intravenous morphine equivalents. The BPI4 was used to document postoperative pain and the resulting limitation of function, while the Quality of Recovery (QoR)5 score was used to document recovery from anesthesia and surgery. Opioid consumption and pain scores were recorded at the completion of surgery, at discharge from the postanesthesia care unit, and at six, 24, and 48 hr following surgery. The following time to recovery milestones were recorded daily: time to first fluid intake, time to first solid intake, time to passage of flatus, and time to first bowel movement. Side effects, including nausea, vomiting, and sedation, were also recorded daily. Patients were contacted seven days following surgery to evaluate pain control and QoR.
A blood sample was drawn one hour following induction of anesthesia to determine serum lidocaine concentration. Samples were frozen and sent to a reference laboratory (St. Michael’s Hospital, Toronto, ON, Canada) to determine serum lidocaine levels. A fluorescence polarization immunoassay was performed using an Abbott TDx analyzer (Abbott Laboratories, Abbott Park, IL, USA).
Data were recorded on standardized forms and transferred to an Excel 2002 spreadsheet (Microsoft Corp, Redmond, WA, USA). At the conclusion of the study, data were cleaned and exported for analysis to SPSS 15 (SPSS Inc, Chicago IL, USA) and SAS 9.1 (SAS Institute, Inc, Cary, NC, USA). Demographic characteristics were described with counts or means and standard deviations. The primary outcome, i.e., date of hospital discharge, was dichotomized to a) discharge on or before POD2 or b) discharge on POD3 or later and was analyzed with a Pearson’s Chi square test. The rationale for this approach is detailed in the sample size section below. Dichotomous secondary outcomes, such as incidence of side effects, were also analyzed with the Pearson’s Chi square test. Recovery milestones and times to discharge and discharge readiness were assessed using the non-parametric Mann Whitney U test and described using medians and interquartile ranges (25th percentile, 75th percentile). Continuous variables, such as morphine consumption, NRS pain, BPI, and QoR scores were described with means and standard deviations (SD) and evaluated with the Student’s two sample t test. Mean differences between the lidocaine and control groups were reported with 95% confidence intervals. Statistical significance was set at α = 0.05. We reported P values directly without adjustment for the multiple secondary outcomes.
The relationship between minimum alveolar concentration (MAC) (dependent variable) and anesthetic depth (bispectral index [BIS]) was explored with mixed effects regression modelling.6 The intercept and slope (BIS) were specified as random coefficients to account for the correlation in repeated measures on the same patient. Differences between the lidocaine and placebo groups were examined by including group and interaction between BIS and group as fixed effects in the model (Appendix).
A retrospective chart review of 47 patients undergoing elective total abdominal hysterectomies under general anesthesia at The Ottawa Hospital (Civic Campus) was completed in May 2006. This review identified a median duration of stay of three days with 21% of all abdominal hysterectomies discharged on POD2. Shifting the median day of discharge to POD2 was considered clinically significant, as reliable discharge of patients on that day would prompt a revision of the clinical pathway defining post-hysterectomy care in our institution. A trial enrolling 42 patients per group was required to document an increase in POD2 discharge from 21-50% with probabilities of two-sided alpha and beta errors of 0.05 and 0.20, respectively.
The review also identified 48-hr morphine consumption of 89.6 (SD 33) mg. A trial enrolling 42 patients per group would identify a 20 mg absolute reduction in 48-hr morphine consumption, with probabilities of two-sided alpha and beta errors of 0.05 and 0.20, respectively.
Sample size was increased to 90 patients to accommodate losses to follow-up and protocol violations.
Results
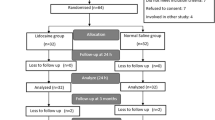
From June 1, 2007 to October 31, 2008, 279 women were assessed for eligibility with 93 consenting to the study. Three patients were excluded after randomization but prior to receiving the intervention, leaving 90 patients in the trial—lidocaine group (n = 44) and control group (n = 46). In-hospital data for the primary outcome was complete for all enrolled patients. Serum lidocaine samples for two patients were misplaced by the hospital laboratory. Five patients (two lidocaine and three control) could not be contacted for follow-up seven days after surgery. Fig. 1 documents patient flow throughout the trial.
We evaluated the distributional properties of all scale variables by means of descriptive statistics, including histograms and normal probability plots. None of the variables had substantial skewness that justified the use of non-parametric methods, and in most cases, means and medians were nearly identical or very similar.
Demographic characteristics were well balanced between study groups (Table 1). There were no differences in length of hospital stay (P = 0.456) or POD2 discharge (P = 0.295) associated with the study groups (Table 2). Time to meet discharge criteria (P = 0.489) and the proportion meeting these criteria on POD2 (P = 0.666) were also similar in both the lidocaine and the control groups (Table 2).
Narcotic consumption (Table 3) and numeric pain rating scores (Table 4) were similar at all time-points. Only one of 44 patients in the lidocaine group was unable to resume oral fluids on the day of surgery compared with nine of 46 patients in the control group (P = 0.01). This earlier resumption of oral intake did not translate into more rapid return of gut function, with patients in both groups reporting a median date of passage of first flatus as POD1 (1,2), P = 0.902. Objective measures of recovery, such as the QoR score and the functional interference score of the BPI, were comparable at all measurement times (Table 5).
Exploratory analysis indicated that the relationship between BIS and MAC might not be linear, so a quadratic term (BIS2) was entered into the model and specified as a third random coefficient. The quadratic relationship between BIS and MAC was significant (P = 0.0001); however, contrary to expectations, lidocaine bore no statistically significant influence on anesthetic requirements—the model indicated no significant difference in the relationship between MAC and BIS between the groups (difference in linear and quadratic terms β = -0.003; P = 0.358 and β = 0.000; P = 0.544, respectively). The relationship between BIS and MAC in the two groups is demonstrated graphically in Fig. 2.
From a safety and tolerability perspective, serum lidocaine levels 60 min following induction (2.63 μg·mL−1 [SD 0.60]) were well below the toxic level (5 μg·mL−1), and no patient experienced signs of local anesthetic toxicity. Subjective symptoms of local anesthetic toxicity (lightheadedness, tinnitus, dysguesia, etc.) were reported by 21 (46%) of the control patients compared with only 11 (26%) of the lidocaine patients (P = 0.049).
Discussion
The results of this trial indicate that intraoperative infusion of intravenous lidocaine did not influence length of hospital stay or discharge readiness following abdominal hysterectomy. Indeed, only 25 of 90 (27.8%) patients enrolled in the study met our a priori study goal of discharge on POD2. Narcotic use and numeric pain rating scores were similar in lidocaine and control groups at all time periods. Side effect profiles and QoR were similar in both groups.
It is interesting to note that 23 additional patients reached discharge fitness on the morning of POD2 but remained in hospital. This gap between discharge readiness and discharge from hospital occurred despite prompts from study personnel indicating that patients had met discharge criteria. Discrepancies between discharge fitness and actual discharge are well described in ambulatory care and most commonly are due to social reasons.7 While not formally measured as an outcome of this trial, we did note that social reasons (expected to stay longer, waited for staple removal, no one at home) were the most common reasons for delay among discharge-ready patients. These findings suggest that a post-hysterectomy care plan aiming for discharge on POD2 may be realistic, but modifications to patient and health care worker expectations of care are required.
The results of the present study contrast those reported in other abdominal surgeries where perioperative lidocaine infusion was associated with improved analgesia and shorter hospital stays.2 What features of this trial contribute to these divergent conclusions? First, the majority of trials included in Marrett’s systematic review2 represented major intestinal or upper abdominal surgery and were associated with longer durations of ileus (range in control group, 22-85 hr) and length of hospital stay (range in control group 3.75-14 days) than noted in our study. It is possible that the shorter durations of stay among women undergoing hysterectomy did not provide sufficient “opportunity” to elicit a lidocaine “benefit”. Second, only three of the eight trials reported the use of narcotic-sparing adjunctive analgesics like acetaminophen or NSAIDs. In the present trial, acetaminophen and celecoxib were given preoperatively and were continued until hospital discharge, perhaps reducing perioperative opioid consumption and limiting a treatment benefit from lidocaine. Lastly, only two of eight trials included in the meta-analysis restricted their lidocaine use to the intraoperative period; three trials extended lidocaine infusions up to four hours postoperatively, and another three trials continued for 24 hr after surgery. By stopping lidocaine at skin closure, it is possible that patients were not receiving drug when they stood to benefit from it. Regardless of the mechanism, it was apparent that intraoperative lidocaine infusion did not improve pain outcomes of time to discharge among patients undergoing hysterectomy.
It is also interesting to note that intravenous infusion of lidocaine failed to reduce the amount of volatile anesthetic required to maintain BIS readings consistent with adequate surgical anesthesia. Lidocaine has a well-known MAC-sparing action. A recent animal study suggests that serum concentrations of lidocaine similar to those noted in the present trial should have decreased MAC by up to 50%.8 Perhaps the BIS is insufficiently sensitive for accurate titration of inhalational anesthetics, and concomitant use of narcotics and benzodiazepines obscured the relationship between lidocaine and MAC. However, it is interesting to note that intravenous lidocaine also failed to reduce volatile anesthetic requirements titrated to BIS in a study directly comparing intravenous and epidural administration of lidocaine.9 Despite similar serum concentrations of lidocaine (2 μg·mL−1) following both intravenous and epidural administration, only those patients given lidocaine in their epidurals showed evidence of anesthetic sparing. Previous research demonstrates that volatile anesthetics exert their immobilizing effect at the spinal level.10 By titrating volatile anesthesia solely to a central measure of anesthetic effect like BIS, it is possible that a MAC-sparing effect of lidocaine active at the spinal cord level may be undetected. Further research to determine the locus of lidocaine’s influence on volatile anesthetic requirements is necessary.
In conclusion, preoperative boluses of lidocaine 1.5 mg·kg−1 followed by continuous intraoperative infusion of lidocaine 3 mg·kg−1·hr−1 until skin closure were not associated with reductions in hospital stay, narcotic consumption, pain scores, or measures of functional recovery following total abdominal hysterectomy. While longer administration of intravenous lidocaine is associated with benefit in other models of abdominal surgery, the restriction of lidocaine to the intraoperative period in lower abdominal operations is not supported.
Notes
Millar WJ. Hysterectomy rates 1981/82 to 1996/97. Statistics Canada. 2001.
References
Philip BK, Reese PR, Burch SP. The economic impact of opioids on postoperative pain management. J Clin Anesth 2002; 14: 354-64.
Marret E, Rolin M, Beaussier M, Bonnet F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg 2008; 95: 1331-8.
Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001; 357: 1191-4.
Mendoza TR, Chen C, Brugger A, et al. The utility and validity of the modified brief pain inventory in a multiple-dose postoperative analgesic trial. Clin J Pain 2004; 20: 357-62.
Myles PS, Hunt JO, Nightingale CE, et al. Development and psychometric testing of a quality of recovery score after general anesthesia and surgery in adults. Anesth Analg 1999; 88: 83-90.
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2nd ed. Cary, NC: SAS Institute, Inc; 2006.
Chung F. Recovery pattern and home-readiness after ambulatory surgery. Anesth Analg 1995; 80: 896-902.
Zhang Y, Laster MJ, Eger EI II, Sharma M, Sonner JM. Lidocaine, MK-801, and MAC. Anesth Analg 2007; 104: 1098-102.
Hodgson PS, Liu SS. Epidural lidocaine decreases sevoflurane requirement for adequate depth of anesthesia as measured by the bispectral index monitor. Anesthesiology 2001; 94: 799-803.
Antognini JF, Carstens E. Macroscopic sites of anesthetic action: brain versus spinal cord. Toxicol Lett 1998; 100-101: 51-8.
Acknowledgements
The authors sincerely thank Dr. Jordan Caveno, Ms. Denise Wozny, Ms. Sharon Finlay, the Chair’s Research Fund team, and the Departments of Anesthesiology and Gynecology at the Ottawa Hospital.
Funding
Trial expenses were funded by the Chair’s Research Fund, Department of Anesthesiology, University of Ottawa. Dr. Bryson was supported by the Ottawa Hospital Anesthesia Alternate Funds Association.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Details of random coefficients model used to examine the relationship between MAC and BIS.
The quadratic model that was used to describe MAC for the kth measurement of the jth patient from the ith treatment is:
where \( \alpha_{i} + \beta_{i} BIC_{k} + \gamma_{i} BIC_{k}^{2} \) is the fixed effects part of the model, and \( a_{ij} + b_{ij} BIC_{k} + c_{ij} BIC_{k}^{2} \) is the random effects part of the model, and \( e_{ijk} \) is the residual party of the model. The random effects are modelled under the following distributional assumptions:
and
MAC = minimum alveolar concentration; BIS = bispectral index.
Rights and permissions
About this article
Cite this article
Bryson, G.L., Charapov, I., Krolczyk, G. et al. Intravenous lidocaine does not reduce length of hospital stay following abdominal hysterectomy. Can J Anesth/J Can Anesth 57, 759–766 (2010). https://doi.org/10.1007/s12630-010-9332-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-010-9332-2