Abstract
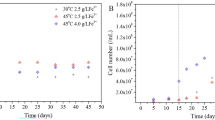
The bioleaching of copper sulphide minerals was investigated by using A. ferrooxidans ATF6. The result shows the preferential order of the minerals bioleaching as djurleite>bornite>pyritic chalcopyrite>covellite>porphyry chalcopyrite. The residues were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). It is indicated that jarosite may not be responsible for hindered dissolution. The elemental sulfur layer on the surface of pyritic chalcopyrite residues is cracked. The compact surface layer of porphyry chalcopyrite may strongly hinder copper extraction. X-ray photoelectron spectroscopy (XPS) further confirms that the passivation layers of covellite, pyritic chalcopyrite, and porphyry chalcopyrite are copper-depleted sulphide Cu4S11, S8, and copper-rich iron-deficient polysulphide Cu4Fe2S9, respectively. The ability of these passivation layers was found as Cu4Fe2S9>Cu4S11>S8>jarosite.
Similar content being viewed by others
References
H.R. Watling, The bioleaching of sulphide minerals with emphasis on copper sulphides: a review, Hydrometallurgy, 84(2006), No.1–2, p.81.
H.L. Ehrlich, Past, present and future of biohydrometallurgy, Hydrometallurgy, 59(2001), No.2–3, p.127.
E.M. Arce and I. González, A comparative study of electrochemical behavior of chalcopyrite, chalcocite and bornite in sulphuric acid solution, Int. J. Miner. Process., 67(2002), No.1–4, p.17.
K.B. Fu, H. Lin, X.L. Mo, Y.B. Dong, and L. Zhou, Study on bioleaching of different types of chalcopyrite, J. Univ. Sci. Technol. Beijing, 33(2011), No.7, p.806.
D. Dreisinger, Copper leaching from primary sulfides: options for biological and chemical extraction of copper, Hydrometallurgy, 83(2006), No.1–4, p.10.
E.M. Córdoba, J.A. Muñoz, M.L. Bláquez, F. González, and A. Ballester, Passivation of chalcopyrite during its chemical leaching with ferric ion at 68°C, Miner. Eng., 22(2009), No.3, p.229.
J.E. Dutrizac, Elemental sulphur formation during the ferric sulphate leaching of chalcopyrite, Can. Metall. Q., 28(1989), No.4, p.337.
C. Klauber, A. Parker, W. Van Bronswijk, and H. Watling, Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy, Int. J. Miner. Process., 62(2001), No.1–4, p.65.
D. Bevilaqua, I. Diéz-Perez, C.S. Fugivara, F. Sanz, A.V. Benedetti, and O. Garcia Jr., Oxidative dissolution of chalcopyrite by Acidithiobacillus ferrooxidans analyzed by electrochemical impedance spectroscopy and atomic force microscopy, Bioelectrochemistry, 64(2004), No.1, p.79.
A.J. Parker, R.L. Paul, and G.P. Power, Electrochemistry of the oxidative leaching of copper from chalcopyrite, J. Electroanal. Chem., 118(1981), p.305.
A. Parker, C. Klauber, A. Kougianos, H.R. Watling, and W. Van Bronswijk, An X-ray photoelectron spectroscopy study of the mechanism of oxidative dissolution of chalcopyrite, Hydrometallurgy, 71(2003), No.1–2, p.265.
A. Sandström, A. Shchukarev, and J. Paul, XPS characterisation of chalcopyrite chemically and bio-leached at high and low redox potential, Miner. Eng., 18(2005), No.5, p.505.
K.A. Third, R. Cord-Ruwisch, and H.R. Watling, Role of iron-oxidizing bacteria in stimulation or inhibition of chalcopyrite bioleaching, Hydrometallurgy, 57(2000), No.3, p.225.
P. Acero, J. Cama, and C. Ayora, Kinetics of chalcopyrite dissolution at pH 3, Eur. J. Mineral., 19(2007), No.2, p.173.
K.B. Fu, H. Lin, X.L. Mo, Y.B. Dong, and H. Wang, Passivation of different genetic types of chalcopyrite bioleaching, J. Cent. South Univ. Sci. Technol., 42(2011), No.11, p.3245.
R.P. Hackl, D.B. Dreisinger, E. Peters, and J.A. King, Passivation of chalcopyrite during oxidative leaching in sulfate media, Hydrometallurgy, 39(1995), No.1–3, p.25.
D.W. Dew, C. Van Buuren, K. Mcewan, and C. Bowker, Bioleaching of base metal sulphide concentrates: A comparison of mesophile and thermopile bacterial cultures, [in] 13th International Biohydrometallurgy Symposium IBS’99, Madrid, 1999, p.229.
F.B. Mateos, I.P. Pérez, and F.C. Mora, The passivation of chalcopyrite subjected to ferric sulphate leaching and its reactivation with metal sulphides, Hydrometallurgy, 19(1987), No.2, p.159.
T. Biegler and M.D. Horne, Electrochemistry of surface oxidation of chalcopyrite, J. Electrochem. Soc., 132(1985), No.6, p.1363.
J.Y. Liu, X.X. Tao, and P. Cai, Study of formation of jarosite mediated by Thiobacillus ferrooxidans in 9K medium, Procedia Earth. Planet. Sci., 1(2009), No.1, p.706.
J.E. Dutrizac, The effect of seeding on the rate of precipitation of ammonium jarosite and sodium jarosite, Hydrometallurgy, 42(1996), No.3 p.293.
B. Meyer, Elemental sulfur, Chem. Rev., 76(1976), No.3, p.367.
C. Klauber, A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution, Int. J. Miner. Process., 86(2008), No.1–4, p.1.
E.C. Todd, D.M. Sherman, and J.A. Purton, Surface oxidation of chalcopyrite (CuFeS2) under ambient atmospheric and aqueous (pH 2–10) conditions: Cu, Fe L- and O K-edge X-ray spectroscopy, Geochim. Cosmochim. Acta, 67(2003), No.12, p.2137.
S.W. Goh, A.N. Buckley, R.N. Lamb, R.A. Rosenberg, and D. Moran, The oxidation states of copper and iron in mineral sulfides, and the oxides formed on initial exposure of chalcopyrite and bornite to air, Geochim. Cosmochim. Acta, 70(2006), No.9, p.2210.
S.L. Harmer, Surface Layer Control for Improved Copper Recovery for Chalcopyrite Leaching [Dissertation], University of South Australia, Adelaide, 2002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, Kb., Lin, H., Mo, Xl. et al. Comparative study on the passivation layers of copper sulphide minerals during bioleaching. Int J Miner Metall Mater 19, 886–892 (2012). https://doi.org/10.1007/s12613-012-0643-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-012-0643-x