Abstract
Objectives
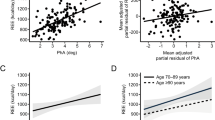
This study aimed to measure resting energy expenditure (REE) in institutionalized old persons and to determine factors possibly related to change in REE as a basis for estimating energy requirements.
Design and Settings
A monocentric cross-sectional study was conducted. Statistical approaches were conducted to determine independent factors associated with REE. Various published predictive equations of REE were compared to our population.
Participants
72 residents of a nursing home, mostly women (80.5%) aged 87.4±6.6 years were included.
Measurements
REE (indirect calorimetry), body composition (bio-impedance analysis), biological and anthropometric data were collected.
Results
Mean REE was 1006±181 kcal/d and was higher in men than in (1227±195 vs. 953±131 kcal/d, p<0.05). According to criteria adapted from the Global Leadership Initiative on Malnutrition consensus, 65.3 % of the institutionalized population were malnourished. In multivariate analysis adjusted on gender and age, REE was positively associated with calorie intake, fat-free mass (FFM), functional abilities (French Autonomie Gérontologie Groupe Iso Ressources scale), and elevated CRP level (> 25 mg/l). Significant differences (p<0.05) appeared between measured REE and predicted REE by using various published equations.
Conclusion
REE of very old nursing home residents is influenced by FFM, calorie intake, functional abilities, and CRP levels and is poorly predicted by classical equations based on age, gender, height, and weight. This suggests a metabolic adaptation to caloric restriction and inflammation and prompts to consider the level of physical activity and muscle loss when assessing caloric requirements in this population.
Similar content being viewed by others
References
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Lond Engl 2017;390:1211–1259. doi:https://doi.org/10.1016/S0140-6736(17)32154-2
Ponti F, Santoro A, Mercatelli D, Gasperini C, Conte M, Martucci M, Sangiorgi L, Franceschi C, Bazzocchi A. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front Endocrinol 2020;10: https://www.frontiersin.org/article/10.3389/fendo.2019.00861 [Accessed April 26, 2022]
Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev 2018;47:123–132. doi:https://doi.org/10.1016/j.arr.2018.07.005
Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr 2001;55:663–672. doi:https://doi.org/10.1038/sj.ejcn.1601198
Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res 1995; 3:73–95. doi:https://doi.org/10.1002/j.1550-8528.1995.tb00124.x
Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001;137:231–243. doi:https://doi.org/10.1067/mlc.2001.113504
Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol Rev 2019; 99:427–511. doi:https://doi.org/10.1152/physrev.00061.2017
Jennings C, Patterson E, Curtis RG, Mazzacano A, Maher CA. Effectiveness of a Lifestyle Modification Program Delivered under Real-World Conditions in a Rural Setting. Nutrients 2021; 13:4040. doi:https://doi.org/10.3390/nu13114040
Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R, Blaauw R, Coats AJS, et al. GLIM criteria for the diagnosis of malnutrition — A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 2019;10:207–217. doi:https://doi.org/10.1002/jcsm.12383
Faxén-Irving G, Basun H, Cederholm T. Nutritional and cognitive relationships and long-term mortality in patients with various dementia disorders. Age Ageing 2005; 34:136–141. doi:https://doi.org/10.1093/ageing/afi023
Wang SY, Fukagawa N, Hossain M, Ooi WL. Longitudinal weight changes, length of survival, and energy requirements of long-term care residents with dementia. J Am Geriatr Soc 1997; 45:1189–1195. doi:https://doi.org/10.1111/j.1532-5415.1997.tb03768.x
Doorduijn AS, de van der Schueren MAE, van de Rest O, de Leeuw FA, Hendriksen HMA, Teunissen CE, Scheltens P, van der Flier WM, Visser M. Energy intake and expenditure in patients with Alzheimer’s disease and mild cognitive impairment: the NUDAD project. Alzheimers Res Ther 2020;12:116. doi:https://doi.org/10.1186/s13195-020-00687-2
Westerterp KR. Physical activity and physical activity induced energy expenditure in humans: measurement, determinants, and effects. Front Physiol 2013;4:90. doi:https://doi.org/10.3389/fphys.2013.00090
Starling RD, Toth MJ, Carpenter WH, Matthews DE, Poehlman ET. Energy requirements and physical activity in free-living older women and men: a doubly labeled water study. J Appl Physiol Bethesda Md 1985 1998;85:1063–1069. doi:https://doi.org/10.1152/jappl.1998.85.3.1063
Energy and protein requirements. Report of a joint FAO/WHO/UNU Expert Consultation. World Health Organ Tech Rep Ser 1985;724:1–206.
Utaka S, Avesani CM, Draibe SA, Kamimura MA, Andreoni S, Cuppari L. Inflammation is associated with increased energy expenditure in patients with chronic kidney disease. Am J Clin Nutr 2005;82:801–805. doi:https://doi.org/10.1093/ajcn/82.4.801
Guerin O, Jeandel C. Rapport de mission: 25 recommandations pour une prise en soins adaptée des patients et des résidents afin que nos établissements demeurent des lieux de vie. https://solidarites-sante.gouv.fr/IMG/pdf/rapport_jeandel-guerin.pdf [Accessed March 16, 2022]
Aguilova L, Sauzéon H, Balland É, Consel C, N’Kaoua B. [AGGIR scale: a contribution to specifying the needs of disabled elders]. Rev Neurol (Paris) 2014; 170:216–221. doi:https://doi.org/10.1016/j.neurol.2014.01.039
Weir JBDB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9. doi:https://doi.org/10.1113/jphysiol.1949.sp004363
Feinberg M, Favier JC, Fondation Française pour la Nutrition P (FRA), Ireland-Ripert J, CIQUAL Centre Informatique sur la Qualité des Aliments P (FRA). Répertoire général des aliments—Table de composition. Versailles: Lavoisier 1995. n. p., 200p. p.
Pereira J, Ribeiro A, Ferreira-Coimbra J, Barroso I, Guimarães J-T, Bettencourt P, Lourenço P. Is there a C-reactive protein value beyond which one should consider infection as the cause of acute heart failure? BMC Cardiovasc Disord 2018;18:40. doi:https://doi.org/10.1186/s12872-018-0778-4
Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A 1918;4:370–373.
Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–247. doi:https://doi.org/10.1093/ajcn/51.2.241
Lührmann PM, Herbert BM, Krems C, Neuhäuser-Berthold M. A new equation especially developed for predicting resting metabolic rate in the elderly for easy use in practice. Eur J Nutr 2002;41:108–113. doi:https://doi.org/10.1007/s003940200016
Altman DG. Practical Statistics for Medical Research. CRC Press 1990;630 p.
Leung K-CW, Sum K-WR, Yang Y-J. Patterns of Sedentary Behavior among Older Adults in Care Facilities: A Scoping Review. Int J Environ Res Public Health 2021; 18:2710. doi:https://doi.org/10.3390/ijerph18052710
Cooper JA, Manini TM, Paton CM, Yamada Y, Everhart JE, Cummings S, Mackey DC, Newman AB, Glynn NW, Tylavsky F, et al. Longitudinal change in energy expenditure and effects on energy requirements of the elderly. Nutr J 2013;12:73. doi:https://doi.org/10.1186/1475-2891-12-73
Derumeaux-Burel H, Meyer M, Morin L, Boirie Y. Prediction of resting energy expenditure in a large population of obese children. Am J Clin Nutr 2004;80:1544–1550. doi:https://doi.org/10.1093/ajcn/80.6.1544
Lazzer S, Boirie Y, Montaurier C, Vernet J, Meyer M, Vermorel M. A weight reduction program preserves fat-free mass but not metabolic rate in obese adolescents. Obes Res 2004;12:233–240. doi:https://doi.org/10.1038/oby.2004.30
Hanaoka BY, Zhao J, Heitman K, Khan F, Jarjour W, Volek J, Brock G, Gower BA. Interaction effect of systemic inflammation and modifiable rheumatoid cachexia risk factors on resting energy expenditure in patients with rheumatoid arthritis. JCSM Clin Rep 2022;7:12–23. doi:https://doi.org/10.1002/crt2.45
Buttgereit F, Burmester GR, Brand MD. Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunol Today 2000;21:192–199. doi:https://doi.org/10.1016/s0167-5699(00)01593-0
Lacourt TE, Vichaya EG, Chiu GS, Dantzer R, Heijnen CJ. The High Costs of Low-Grade Inflammation: Persistent Fatigue as a Consequence of Reduced Cellular-Energy Availability and Non-adaptive Energy Expenditure. Front Behav Neurosci 2018;12: https://www.frontiersin.org/article/10.3389/fnbeh.2018.00078 [Accessed April 26, 2022]
Hashizume N, Tanaka Y, Yoshida M, Fukahori S, Ishii S, Saikusa N, Masui D, Higashidate N, Sakamoto S, Tsuruhisa S, et al. Resting energy expenditure prediction using bioelectrical impedance analysis in patients with severe motor and intellectual disabilities. Brain Dev 2019;41:352–358. doi:https://doi.org/10.1016/j.braindev.2018.11.003
Bosy-Westphal A, Kossel E, Goele K, Later W, Hitze B, Settler U, Heller M, Glüer C-C, Heymsfield SB, Müller MJ. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. Am J Clin Nutr 2009;90:993–1001. doi:https://doi.org/10.3945/ajcn.2008.27402
Most J, Redman LM. Impact of calorie restriction on energy metabolism in humans. Exp Gerontol 2020;133:110875. doi:https://doi.org/10.1016/j.exger.2020.110875
Wang H, Ye J. Regulation of energy balance by inflammation: common theme in physiology and pathology. Rev Endocr Metab Disord 2015;16:47–54. doi:https://doi.org/10.1007/s11154-014-9306-8
Donini LM, Stephan BCM, Rosano A, Molfino A, Poggiogalle E, Lenzi A, Siervo M, Muscaritoli M. What Are the Risk Factors for Malnutrition in Older-Aged Institutionalized Adults? Nutrients 2020;12:2857. doi:https://doi.org/10.3390/nu12092857
Bell CL, Lee ASW, Tamura BK. Malnutrition in the nursing home. Curr Opin Clin Nutr Metab Care 2015;18:17–23. doi:https://doi.org/10.1097/MCO.0000000000000130
Gregori D, Ocagli H, Lanera C, Gallipoli S, Lorenzoni G. Systematic Review of Equations for Estimating Energy Requirement in the Elderly: Results and Future Perspectives. Curr Dev Nutr 2020;4:28. doi:https://doi.org/10.1093/cdn/nzaa040_028
Karlsson M, Olsson E, Becker W, Karlström B, Cederholm T, Sjögren P. Ability to predict resting energy expenditure with six equations compared to indirect calorimetry in octogenarian men. Exp Gerontol 2017;92:52–55. doi:https://doi.org/10.1016/j.exger.2017.03.013
Ocagli H, Lanera C, Azzolina D, Piras G, Soltanmohammadi R, Gallipoli S, Gafare CE, Cavion M, Roccon D, Vedovelli L, et al. Resting Energy Expenditure in the Elderly: Systematic Review and Comparison of Equations in an Experimental Population. Nutrients 2021;13:458. doi:https://doi.org/10.3390/nu13020458
Cioffi I, Marra M, Pasanisi F, Scalfi L. Prediction of resting energy expenditure in healthy older adults: A systematic review. Clin Nutr 2021;40:3094–3103. doi:https://doi.org/10.1016/j.clnu.2020.11.027
Cegielski J, Wilkinson DJ, Brook MS, Boereboom C, Phillips BE, Gladman JFR, Smith K, Atherton PJ. Combined in vivo muscle mass, muscle protein synthesis and muscle protein breakdown measurement: a ‘Combined Oral Stable Isotope Assessment of Muscle (COSIAM)’ approach. GeroScience 2021;43:2653–2665. doi:https://doi.org/10.1007/s11357-021-00386-2
Holmes CJ, Racette SB. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021;13:2493. doi:https://doi.org/10.3390/nu13082493
Lupoli L, Sergi G, Coin A, Perissinotto E, Volpato S, Busetto L, Inelmen EMEM, Enzi G. Body composition in underweight elderly subjects: reliability of bioelectrical impedance analysis. Clin Nutr Edinb Scotl 2004;23:1371–1380. doi:https://doi.org/10.1016/j.clnu.2004.05.005
Ordovas JM, Berciano S. Personalized nutrition and healthy aging. Nutr Rev 2020; 78:58–65. doi:https://doi.org/10.1093/nutrit/nuaa102
Acknowledgments and contribution
We thank the patients for their valuable contribution to the study. No specific grant supported this study. CL contributed to data analysis, interpretation, and manuscript preparation. HDB contributed to the design of the study, data collection, data analysis and interpretation, and manuscript preparation. CG contributed to data collection, interpretation, and manuscript preparation. BP contributed to data analysis and manuscript preparation. YB was responsible for the study conception, data interpretation, and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest: None of the authors had any financial or personal conflicts of interest with this research.
Ethical standards: Each patient completed an information and consent form. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as revised in 1983 and received approval from the institution’s human research committee (Comité de Protection des Personnes Sud-Est VI). The study complies with the current French laws concerning clinical research.
Electronic supplementary material
Supplementary Figure
: Bland and Altman analysis to compare REE estimation with WHO equation to measured REE (MJ/d).
Supplementary Table
: Characteristics of malnourished and well-nourished frail old populations
Rights and permissions
About this article
Cite this article
Lahaye, C., Derumeaux-Burel, H., Guillet, C. et al. Determinants of Resting Energy Expenditure in Very Old Nursing Home Residents. J Nutr Health Aging 26, 872–878 (2022). https://doi.org/10.1007/s12603-022-1837-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-022-1837-1