Abstract
Objectives
The efficacy, safety and disease-modification of tramiprosate (homotaurine)were investigated in a recently completed large-scale Phase III clinical study in patients with mild to moderate Alzheimer’s disease (AD), the Alphase study. Disease-modification was assessed using longitudinal volumetric MRI (vMRI) measurements of the hippocampus in a subgroup of patients. The present study describes the vMRI, cognitive and clinical results obtained in this subgroup.
Design
Multi-center, double-blind, randomized, placebocontrolled study in a subset of the 1052 patients of the Alphase study.
Setting
51 vMRI investigative sites in the United States and Canada.
Participants
A total of 508 patients underwent vMRI scanning. Of these, 312 provided scan pairs for assessing hippocampus volume changes and were included in the analyses.
Interventions
Patients were randomized to receive Placebo BID (n = 109), tramiprosate 100 mg BID (n = 103), or tramiprosate 150 mg BID (n = 100) for 78 weeks.
Measurements
Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) and Clinical Dementia Rating-Sum-of-boxes CDR-SB assessments were conducted at Baseline and at Weeks 13, 26, 39, 52, 65 and 78. Exploratory analyses were performed using similar First and Final mixed-effects repeated-measures models that were used for the analysis of the entire patient dataset.
Results
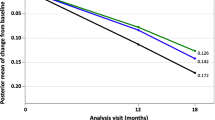
Psychometric score results showed numerical trends in favour of tramiprosate that did not reach statistical significance. While there were no statistically significant group differences in hippocampus volume using the First modeling approach, a significant dose-response reduction in hippocampus volume change was found in the Final models. Moreover, there was a marginally significant overall treatment main effect and a significant slope difference in favour of tramiprosate according to the Final model analysis of the ADAS-cog scores. ADAS-cog scores analyzed according to this model also revealed differences in favor of the tramiprosate 150 mg group at weeks 26 and 52, with marginally significant differences at Weeks 13 and 39. Slope analyses of ADAS-cog score changes showed significant differences in favor of the 150 mg BID group, and when both active groups were combined, in comparison to the placebo group. No between-group differences with respect to changes to each visit in the CDR-SB were observed with either modeling approach. Although there was a similar dose-response relationship observed in the hippocampus volume and ADAS-cog Final model analyses, the overall changes in psychometric scores and hippocampus volume were not significantly correlated.
Conclusion
Exploratory analysis of the vMRI subgroup suggests that tramiprosate slows hippocampal atrophy, and reveals some evidence of a beneficial effect on cognition. The clinical validity of the vMRI biomarker is discussed.
Similar content being viewed by others
References
Canaveri L, Abramov A, & Duchen MR Toxicity of amyloid B peptide: Tales of calcium, mitochondria, and oxidative stress. Neurochem Res 2004; 29: 637–650.
Goshe KM, Mortimer JA, Smith CD, et al. Hippocampal volume as an index of Azheimer neuropathology: findings from the Nun study. Neurology 2002; 58: 1476–1482
Haroutunian V, Perl DP, Purohit DP, et al. Regional distribution of neuritic plaques in the non-demented elderly and subjects with very mild Alzheimer disease. Arch. Neurol 1998; 55: 1185–1191.
Goshe KM, Mortimer JA, Smith CD, et al. Hippocampal volume as an index of Azheimer neuropathology: findings from the Nun study. Neurology 2002; 58: 1476–1482
Convit A, de Leon MJ, Golomb J, et al. Hippocampal atrophy in early Alzheimer’s disease: anatomic specificity and validation. Psychiatric Quarterly 1993; 64: 371–387.
Csernansky JG, Wang L, Joshi S, et al. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Neurology 2000; 55: 1636–1643.
Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiology of Aging 2001; 22: 747–754.
Du AT, Schuff N, Amend D, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry 2001; 71: 441–447.
Du AT, Schuff N, Kramer JH, et al. Higher Atrophy Rate of Entorhinal Cortex than Hippocampus in AD. Neurology 2004; 62: 422–427.
Krasuski JS, Alexander GE, Horwitz B, et al. Relation of medial temporal lobe volumes to age and memory function in non-demented adults with Down’s syndrome: implications for the prodromal phase of Alzheimer’s disease. American Journal of Psychiatry 2002; 159: 74–81.
Mega MS, Small GW, Xu ML, et al. Hippocampal atrophy in persons with age-associated memory impairment: volumetry within a common space. Psychosomatic Medicine 2002; 64; 487–492.
Mu Q, Xie J, Wen Z, et al. A quantitative MR study of the hippocampal formation, the amygdale, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. American Journal of Neuroradiology 1999;20:207–211.
Xu Y, Jack CRJ, O’Brien PC, et al. Usefulness of MRI measures of entrorhinal versus hippocampus in AD. Neurology 2000; 14: 531–545.
Petersen RC, Jack CR Jr, Xu YC, et al. Memory and MRI-based hippocampal volumes in aging and AD. Neurology 2000; 54: 581–587.
Persson J, Nyberg L, Lind J, et al. Structure-function correlates of cognitive decline in aging. Cereb Cortex 2006; 16: 907–915.
Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006; 67: 834–842.
Citron, M. Strategies for disease modification in Alzheimer’s disease. Nature Reviews 2004; 5: 677–685.
Gervais F, Paquette J, Morissette C, et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging 2007; 28:537–547.
Krzywkowski P, Sebastiani G, Williams S, et al. Tramiprosate Prevents Amyloid Beta-induced Inhibition of Long-term Potentiation in Rat Hippocampal Slices. 8th International Conference AD/PD, Salzburg, Austria, March 14–18. 2007.
Azzi M, Morissette C, Fallon L, et al. Involvement of both GABA-dependent and — independent pathways in tramiprosate neuroprotective effects against amyloid-beta toxicity. 8th International Conference AD/PD, Salzburg, Austria, March 14–18.2007.
Aisen PS, Saumier D, Briand R, et al. A Phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology 2006; 67:1757–1763.
Aisen PS, Gauthier S, Ferris SH, et al. Tramiprosate in Mild to Moderate Alzheimer’s Disease: a Randomized, Double-blind, Placebo-controlled, Multi-centre study (the Alphase study). Submitted.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition, Text Revision. 2000. Washington, DC, American Psychiatric Association, 154–158.
McKhann G, Drachman D, Folskein M, et al. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Word Group under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34: 939–944.
Folstein MF, Folstein SE and McHugh PR. “Mini-Mental State” A Practical Method for Grading the Cognitive State of Patients for the Clinician. J Psychiat Res 1975; 12: 189–198.
Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research 1983; 17: 37–49.
Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. JEBS 1998; 24: 323–355.
Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Springer, New York, 2000. p. 568
Littell RC, Milliken GA, Stroup WW, et al. SAS for mixed models. SAS Institute, Cary NC, 2006, pp. 813
West BT, Welch KB, Galecki AT. Linear mixed models. A practical guide using statistical software. Chapman & Hall/CRC, Boca Raton FL, 2007. pp. 353
Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics 2007; 43: 913–928.
Stern RG, Mohs RC, Davidson M, et al. A longitudinal study of Alzheimer’s disease: measurement, rate, and predictors of cognitive deterioration. American Journal of Psychiatry 1994; 151: 390–396.
Author information
Authors and Affiliations
Corresponding author
Additional information
FOR THE ALPHASE GROUP
An erratum to this article is available at http://dx.doi.org/10.1007/s12603-010-0015-z.
Rights and permissions
About this article
Cite this article
Gauthier, S., Aisen, P.S., Ferris, S.H. et al. Effect of tramiprosate in patients with mild-to-moderate alzheimer’s disease: Exploratory analyses of the MRI sub-group of the alphase study. J Nutr Health Aging 13, 550–557 (2009). https://doi.org/10.1007/s12603-009-0106-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-009-0106-x