Abstract
Objective
To better understand the seemingly contradictory plasma β-amyloid (Aβ) results in Alzheimer’s disease (AD) patients by using a newly developed plasma Aβ assay, the INNO-BIA plasma Aβ forms, in a multicenter study.
Methods
A combined retrospective analysis of plasma Aβ isoforms on mild cognitive impairment (MCI) from three large cross-sectional studies involving 643 samples from the participating German and Swedish centers.
Results
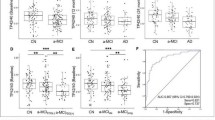
Detection modules based on two different amino (N)-terminal specific Aβ monoclonal antibodies demonstrated that Aβ in plasma could be reliable quantified using a sandwich immunoassay technology with high precision, even for low Aβ42 plasma concentrations. Aβ40 and Aβ42 concentrations varied consistently with the ApoE genotype, while the Aβ42/Aβ40 ratio did not. Irrespective of the decrease of the Aβ42/Aβ40 ratio with age and MMSE, this parameter was strongly associated with AD, as defined in this study by elevated hyperphosphorylated (P-tau181P) levels in cerebrospinal fluid (CSF).
Conclusion
A highly robust assay for repeatedly measuring Aβ forms in plasma such as INNO-BIA plasma Aβ forms might be a useful tool in a future risk assessment of AD.
Similar content being viewed by others
References
Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 2006; 5(3):228–234.
Diniz BS, Pinto JA, Jr., Forlenza OV. Do CSF total tau, phosphorylated tau, and beta-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer’s disease? A systematic review and meta-analysis of the literature. World J Biol Psychiatry 2007; 9(3):172–182.
Engelborghs S, De vreese K, Van de Casteele T., Vanderstichele H, Van Everbroeck B, Cras P et al. Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging 2007; 29(8):1143–1159.
Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH et al. Decreased beta-amyloid1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 2003; 289(16):2094–2103.
Zetterberg H, Blennow K. Plasma Abeta in Alzheimer’s disease—up or down? Lancet Neurol 2006; 5(8):638–639.
Fullwood NJ, Hayashi Y, Allsop D. Plasma amyloid-beta concentrations in Alzheimer’s disease: an alternative hypothesis. Lancet Neurol 2006; 5(12):1000–1001.
Oprisiu R, Serot JM, Godefroy O, Black SE, Fournier A. Plasma amyloid-beta concentrations in Alzheimer’s disease: an alternative hypothesis. Lancet Neurol 2006; 5(12):1001–1002.
Ertekin-Taner N, Younkin LH, Yager DM, Parfitt F, Baker MC, Asthana S et al. Plasma amyloid ta protein is elevated in late-onset Alzheimer disease families. Neurology 2008; 70(8):596–606.
Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol 2007; 64(3):354–362.
Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, Londos E et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging 2008; in press.
Kornhuber J, Schmidtke K, Frolich L, Perneczky R, Wolf S, Hampel H et al. Early and differential diagnosis of dementia and MCI: design and cohort baseline characteristics of the German Competence Net Dementias. Dementia Geriatr. Cogn. Disorders in press.
Nordlund A, Rolstad S, Hellstrom P, Sjogren M, Hansen S, Wallin A. The Goteborg MCI study: mild cognitive impairment is a heterogeneous condition. J Neurol Neurosurg Psychiatry 2005; 76(11):1485–1490.
Lewczuk P, Kornhuber J, Wiltfang J. The German Competence Net Dementias: standard operating procedures for the neurochemical dementia diagnostics. J Neural Transm 2006; 113(8):1075–1080.
Lewczuk P, Kornhuber J, Vanderstichele H, Vanmechelen E, Esselmann H, Bibl M et al. Multiplexed quantification of dementia biomarkers in the CSF of patients with early dementias and MCI: A multicenter study. Neurobiol Aging 2008; 29(6):812–818.
Bouwman FH, Schoonenboom SN, van der Flier WM, Van Elk EJ, Kok A, Barkhof F et al. CSF biomarkers and medial temporal lobe atrophy predict dementia in mild cognitive impairment. Neurobiol Aging 2007; 28(7):1070–1074.
Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 2007; 64(3):343–349.
Blennow K, Zetterberg H, Minthon L, Lannfelt L, Strid S, Annas P et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett 2007; 419(1):18–22.
Bouwman FH, van der Flier WM, Schoonenboom NS, Van Elk EJ, Kok A, Rijmen F et al. Longitudinal changes of CSF biomarkers in memory clinic patients. Neurology 2007; 69(10):1006–1011.
Zetterberg H, Pedersen M, Lind K, Svensson M, Rolstad S, Eckerstrom C et al. Intraindividual stability of CSF biomarkers for Alzheimer’s disease over two years. J Alzheimers Dis 2007; 12(3):255–260.
Lewczuk P, Esselmann H, Bibl M, Beck G, Maler JM, Otto M et al. Tau protein phosphorylated at threonine 181 in CSF as a neurochemical biomarker in Alzheimer’s disease: original data and review of the literature. J Mol Neurosci 2004; 23(1–2):115–122.
van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Abeta(1–40) and Abeta(1–42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol 2006; 5(8):655–660.
Xu W, Kawarabayashi T, Matsubara E, Deguchi K, Murakami T, Harigaya Y et al. Plasma antibodies to Abeta40 and Abeta42 in patients with Alzheimer’s disease and normal controls. Brain Res 2008; 1219:169–179.
Schupf N, Tang MX, Fukuyama H, Manly J, Andrews H, Mehta P et al. Peripheral Abeta subspecies as risk biomarkers of Alzheimer’s disease. Proc Nat Acad Sci 2008; 105(37):14052–14057.
Abdullah L, Paris D, Luis C, Quadros A, Parrish J, Valdes L et al. The influence of diagnosis, intra- and inter-person variability on serum and plasma Abeta levels. Neurosci Lett 2007; 428(2–3):53–58.
Author information
Authors and Affiliations
Consortia
Additional information
KND study group: Charité, Berlin, Campus Benjamin Franklin: Heuser, I., Department of Psychiatry, University Bonn: Maier, W., Department of Psychiatry, University Düsseldorf: Luckhaus, C., Department of Psychiatry, University Göttingen: Rüther, E., Center for Geriatric Medicine and Gerontology, University Hospital Freiburg: Hüll, M., Department of Psychiatry, University Hamburg: Jahn,H., Department of Psychiatry, University Leipzig: Gertz, H.J., Central Central Institute of Mental Health, Mannheim: Frölich, L., Department of Psychiatry, Ludwig Maximilian University Munich, Hampel,H., Department of Psychiatry and Psychotherapy, Technical University of Munich: Pernetzki, R.
These authors contributed equally to this study
Rights and permissions
About this article
Cite this article
Blennow, K., De Meyer, G., Hansson, O. et al. Evolution of Aβ42 and Aβ40 levels and Aβ42/Aβ40 ratio in plasma during progression of Alzheimer’s disease: A multicenter assessment. J Nutr Health Aging 13, 205–208 (2009). https://doi.org/10.1007/s12603-009-0059-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-009-0059-0