Abstract
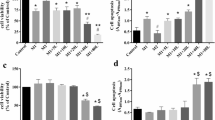
Information regarding cellular anti-senescence attributes of probiotic bacteria vis-à-vis modulation of senescence-associated secretory phenotype (SASP) and mTOR signaling is very limited. The present study assessed anti-senescence potential of secretory metabolites of probiotic Lactobacillus fermentum (Lact. fermentum) using H2O2-induced model of senescence in 3T3-L1 preadipocytes. Application of H2O2-induced cellular senescence characterized by increased cell size and SA-β-gal activity, activation of SASP and reactive oxygen species (ROS), DNA damage response and induction of cell cycle inhibitors (p53/p21WAF1/p16INK4a). Further, a robust stimulation of the PI3K/Akt/mTOR pathway and AMPK signaling was also observed in H2O2-treated cells. However, exposure of cells to cell-free supernatant of Lact. fermentum significantly attenuated phosphorylation of PI3K/Akt/mTOR pathway and alleviated senescence markers p53, p21WAF1, SA-β-gal, p38MAPK, iNOS, cox-2, ROS, NF-κB, and DNA damage response. These results provide evidence that secretory metabolites of Lact. fermentum can mitigate the development as well as severity of stress-induced senescence thereby indicating its utility for use as anti-aging or age-delaying agent.









Similar content being viewed by others
References
Bhatia-Dey N, Kanherkar RR, Stair SE, Makarev EO, Csoka AB (2016) Cellular senescence as the causal nexus of aging. Front Genet 7:13. https://doi.org/10.3389/fgene.2016.00013
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong JA, Saltness R, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM (2016) Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530(7589):184–189. https://doi.org/10.1038/nature16932
Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D (2016) ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxidative Med Cell Longev 2016:1–18. https://doi.org/10.1155/2016/3565127
Sharma R, Kapila R, Kapila S (2013) Probiotics as anti-immunosenescence agents. Food Rev Int 29(2):201–216. https://doi.org/10.1080/87559129.2012.751547
Markowiak P, Slizewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9(9):1021. https://doi.org/10.3390/nu9091021
Kerry RG, Patra JK, Gouda S, Park Y, Shin H-S, Das G (2018) Benefaction of probiotics for human health: a review. J Food Drug Anal 26(3):927–939. https://doi.org/10.1016/j.jfda.2018.01.002
Ershler WB, Keller ET (2000) Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 51(1):245–270. https://doi.org/10.1146/annurev.med.51.1.245
Malaguarnera G, Leggio F, Vacante M, Motta M, Giordano M, Biondi A, Basile F, Mastrojeni S, Mistretta A, Malaguarnera M, Toscano MA, Salmeri M (2011) Probiotics in the gastrointestinal diseases of the elderly. J Nutr Health Aging 16(4):402–410. https://doi.org/10.1007/s12603-011-0357-1
Sharma R, Kapila R, Kapasiya M, Saliganti V, Dass G, Kapila S (2014) Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutr Res 34(11):968–981. https://doi.org/10.1016/j.nutres.2014.09.006
Jeong J-J, Kim K-A, Jang S-E, Woo J-Y, Han MJ, Kim D-H (2015) Orally administrated Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa B signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS One 10(2):e0116533. https://doi.org/10.1371/journal.pone.0116533
Landete JM, Gaya P, Rodríguez E, Langa S, Peirotén Á, Medina M, Arqués JL (2017) Probiotic bacteria for healthier aging: immunomodulation and metabolism of phytoestrogens. Biomed Res Int 2017:1–10. https://doi.org/10.1155/2017/5939818
Blagosklonny MV (2008) Aging: ROS or TOR. Cell Cycle 7(21):3344–3354. https://doi.org/10.4161/cc.7.21.6965
Johnson SC, Rabinovitch PS, Kaeberlein M (2013) mTOR is a key modulator of ageing and age-related disease. Nature 493(7432):338–345. https://doi.org/10.1038/nature11861
Kennedy BK, Lamming DW (2016) The mechanistic target of rapamycin: the grand conducTOR of metabolism and aging. Cell Metab 23(6):990–1003. https://doi.org/10.1016/j.cmet.2016.05.009
Kumar R, Sharma A, Kumari A, Gulati A, Padwad Y, Sharma R (2018) Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology 20:171–189. https://doi.org/10.1007/s10522-018-9785-1
Kumari M, Swarnkar MK, Kumar S, Singh AK, Gupta M (2015) Genome sequence of a potential probiotic strain, Lactobacillus fermentum HFB3, isolated from a human gut. Genome Announc 3(6). https://doi.org/10.1128/genomea.01296-15
Sharma R, Kumari M, Kumari A, Sharma A, Gulati A, Gupta M, Padwad Y (2019) Diet supplemented with phytochemical epigallocatechin gallate and probiotic Lactobacillus fermentum confers second generation synbiotic effects by modulating cellular immune responses and antioxidant capacity in aging mice. Eur J Nutr. https://doi.org/10.1007/s00394-018-01890-6
Nanjundaiah YS, Wright DA, Baydoun AR, O’Hare WT, Ali Z, Khaled Z, Sarker MH (2016) Lactobacillus rhamnosus GG conditioned media modulates acute reactive oxygen species and nitric oxide in J774 murine macrophages. Biochem Biophys Rep 6:68–75. https://doi.org/10.1016/j.bbrep.2016.03.003
Sharma A, Joshi R, Kumar S, Sharma R, Rajneesh, Padwad Y, Gupta M (2018) Prunus cerasoides fruit extract ameliorates inflammatory stress by modulation of iNOS pathway and Th1/Th2 immune homeostasis in activated murine macrophages and lymphocytes. Inflammopharmacology 26(6):1483–1495. https://doi.org/10.1007/s10787-018-0448-2
Sharma R, Sharma A, Kumari A, Kulurkar PM, Raj R, Gulati A, Padwad YS (2017) Consumption of green tea epigallocatechin-3-gallate enhances systemic immune response, antioxidative capacity and HPA axis functions in aged male swiss albino mice. Biogerontology 18(3):367–382. https://doi.org/10.1007/s10522-017-9696-6
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1006/abio.1976.9999
Fuente M, Miquel J (2009) An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des 15(26):3003–3026. https://doi.org/10.2174/138161209789058110
Wang Z, Wei D, Xiao H (2013) Methods of cellular senescence induction using oxidative stress. In: Biological aging. Humana Press, Totowa, NJ, pp 135–144. https://doi.org/10.1007/978-1-62703-556-9_11
Jeong JJ, Woo JY, Kim KA, Han MJ, Kim DH (2015) Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent memory impairment in Fischer 344 rats. Lett Appl Microbiol 60(4):307–314. https://doi.org/10.1111/lam.12393
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J (2015) Probiotics as potential antioxidants: a systematic review. J Agric Food Chem 63(14):3615–3626. https://doi.org/10.1021/jf506326t
Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Wang Y, Li W (2017) Antioxidant properties of probiotic bacteria. Nutrients 9(5):521. https://doi.org/10.3390/nu9050521
Finamore A, Ambra R, Nobili F, Garaguso I, Raguzzini A, Serafini M (2018) Redox role of Lactobacillus casei Shirota against the cellular damage induced by 2,2′-Azobis (2-Amidinopropane) dihydrochloride-induced oxidative and inflammatory stress in enterocytes-like epithelial cells. Front Immunol 9:1131. https://doi.org/10.3389/fimmu.2018.01131
De Marco S, Sichetti M, Muradyan D, Piccioni M, Traina G, Pagiotti R, Pietrella D (2018) Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid Based Complement Alternat Med 2018:1–12. https://doi.org/10.1155/2018/1756308
Borodkina AV, Deryabin PI, Giukova AA, Nikolsky NN (2018) “Social Life” of senescent cells: what is SASP and why study it? Acta Nat 10(1):4–14
Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, Kogan SC, Lowe SW (2011) Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev 25(20):2125–2136. https://doi.org/10.1101/gad.17276711
Salminen A, Kauppinen A, Kaarniranta K (2012) Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal 24(4):835–845. https://doi.org/10.1016/j.cellsig.2011.12.006
Birch J, Passos JF (2017) Targeting the SASP to combat ageing: mitochondria as possible intracellular allies? Bioessays 39(5):1600235. https://doi.org/10.1002/bies.201600235
Osorio FG, Barcena C, Soria-Valles C, Ramsay AJ, de Carlos F, Cobo J, Fueyo A, Freije JMP, Lopez-Otin C (2012) Nuclear lamina defects cause ATM-dependent NF-κB activation and link accelerated aging to a systemic inflammatory response. Genes Dev 26(20):2311–2324. https://doi.org/10.1101/gad.197954.112
Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SLF, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA, von Zglinicki T (2014) Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 5(1). https://doi.org/10.1038/ncomms5172
Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran Samuel C, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez P-Y, Benz CC, Kapahi P, Nelson PS, Campisi J (2015) MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17(8):1049–1061. https://doi.org/10.1038/ncb3195
Stout MB, Steyn FJ, Jurczak MJ, Camporez J-PG, Zhu Y, Hawse JR, Jurk D, Palmer AK, Xu M, Pirtskhalava T, Evans GL, de Souza SR, Frank AP, White TA, Monroe DG, Singh RJ, Casaclang-Verzosa G, Miller JD, Clegg DJ, LeBrasseur NK, von Zglinicki T, Shulman GI, Tchkonia T, Kirkland JL (2016) 17α-Estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J Gerontol A Biol Sci Med Sci 72(1):3–15. https://doi.org/10.1093/gerona/glv309
Dasari A, Bartholomew JN, Volonte D, Galbiati F (2006) Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res 66(22):10805–10814. https://doi.org/10.1158/0008-5472.can-06-1236
Harada G, Neng Q, Fujiki T, Katakura Y (2014) Molecular mechanisms for the p38-induced cellular senescence in normal human fibroblast. J Biochem 156(5):283–290. https://doi.org/10.1093/jb/mvu040
Xu Y, Li N, Xiang R, Sun P (2014) Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem Sci 39(6):268–276. https://doi.org/10.1016/j.tibs.2014.04.004
Borodkina AV, Shatrova AN, Nikolsky NN, Burova EB (2016) The role of p38 MAP-kinase in stress-induced senescence of human endometrium-derived mesenchymal stem cells. Cell Tissue Biol 10(5):365–371. https://doi.org/10.1134/s1990519x16050023
Takanashi N, Tomosada Y, Villena J, Murata K, Takahashi T, Chiba E, Tohno M, Shimazu T, Aso H, Suda Y, Ikegami S, Itoh H, Kawai Y, Saito T, Alvarez S, Kitazawa H (2013) Advanced application of bovine intestinal epithelial cell line for evaluating regulatory effect of lactobacilli against heat-killed enterotoxigenic Escherichia coli-mediated inflammation. BMC Microbiol 13(1):54. https://doi.org/10.1186/1471-2180-13-54
Yan F, Polk DB (2002) Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 277(52):50959–50965. https://doi.org/10.1074/jbc.m207050200
Blagosklonny MV (2010) Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle 9(16):3171–3176. https://doi.org/10.4161/cc.9.16.13120
Carroll B, Korolchuk VI (2017) Dysregulation of mTORC1/autophagy axis in senescence. Aging (Albany NY) 9(8):1851–1852. https://doi.org/10.18632/aging.101277
Nacarelli T, Azar A, Sell C (2015) Aberrant mTOR activation in senescence and aging: a mitochondrial stress response? Exp Gerontol 68:66–70. https://doi.org/10.1016/j.exger.2014.11.004
Nogueira V, Park Y, Chen C-C, Xu P-Z, Chen M-L, Tonic I, Unterman T, Hay N (2008) Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 14(6):458–470. https://doi.org/10.1016/j.ccr.2008.11.003
Swami M (2008) Akt: a double-edged sword. Nat Rev Cancer 9(2):76–77. https://doi.org/10.1038/nrc2586
Bent EH, Gilbert LA, Hemann MT (2016) A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes Dev 30(16):1811–1821. https://doi.org/10.1101/gad.284851.116
Taherian-Esfahani Z, Abedin-Do A, Nouri Z, Mirfakhraie R, Ghafouri-Fard S, Motevaseli E (2016) Lactobacilli differentially modulate mTOR and Wnt/ β-catenin pathways in different cancer cell lines. Iran J Cancer Prev 9(3). https://doi.org/10.17795/ijcp-5369
Fu L, Peng J, Zhao S, Zhang Y, Su X, Wang Y (2017) Lactic acid bacteria-specific induction of CD4+Foxp3+T cells ameliorates shrimp tropomyosin-induced allergic response in mice via suppression of mTOR signaling. Sci Rep 7(1):1987. https://doi.org/10.1038/s41598-017-02260-8
Hwang JW, Baek Y-M, Yang KE, Yoo H-S, Cho C-K, Lee Y-W, Park J, Eom C-Y, Lee Z-W, Choi J-S, Jang I-S (2012) Lactobacillus casei extract induces apoptosis in gastric cancer by inhibiting NF-κB and mTOR-mediated signaling. Integr Cancer Ther 12(2):165–173. https://doi.org/10.1177/1534735412442380
Aw W, Fukuda S (2018) Understanding the role of the gut ecosystem in diabetes mellitus. J Diabetes Investig 9(1):5–12. https://doi.org/10.1111/jdi.12673
de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS (2017) Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care 40(1):54–62. https://doi.org/10.2337/dc16-1324
Tomasello B, Malfa G, Galvano F, Renis M (2011) DNA damage in normal-weight obese syndrome measured by comet assay. Mediterr J Nutr Metab 4:99–104. https://doi.org/10.1007/s12349-010-0035-6
Sharon G, Garg N, Debelius J, Knight R, Dorrestein Pieter C, Mazmanian Sarkis K (2014) Specialized metabolites from the microbiome in health and disease. Cell Metab 20(5):719–730. https://doi.org/10.1016/j.cmet.2014.10.016
Wu R, Jeffrey M, Johnson-Henry K, Green-Johnson J, Sherman P (2016) Impact of prebiotics, probiotics and gut derived metabolites on host immunity. LymphoSign J 4(1):1–24. https://doi.org/10.14785/lymphosign-2016-0012
Hemarajata P, Versalovic J (2012) Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Ther Adv Gastroenterol 6(1):39–51. https://doi.org/10.1177/1756283x12459294
Calabrese V, Dattilo S, Petralia A, Parenti R, Pennisi M, Koverech G, Calabrese V, Graziano A, Monte I, Maiolino L, Ferreri T, Calabrese EJ (2015) Analytical approaches to the diagnosis and treatment of aging and aging-related disease: redox status and proteomics. Free Radic Res 49(5):511–524. https://doi.org/10.3109/10715762.2015
Park J-E, Oh S-H, Cha Y-S (2013) Lactobacillus plantarum LG42 isolated from gajami sik-hae inhibits adipogenesis in 3T3-L1 adipocyte. Biomed Res Int 2013:1–7. https://doi.org/10.1155/2013/460927
Lee E, Jung S-R, Lee S-Y, Lee N-K, Paik H-D, Lim S-I (2018) Lactobacillus plantarum strain ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients 10(5):643. https://doi.org/10.3390/nu10050643
Zoico E, Di Francesco V, Olioso D, Fratta Pasini AM, Sepe A, Bosello O, Cinti S, Cominacini L, Zamboni M (2009) In vitro aging of 3T3-L1 mouse adipocytes leads to altered metabolism and response to inflammation. Biogerontology 11(1):111–122. https://doi.org/10.1007/s10522-009-9236-0
Trabucco Sally E, Zhang H (2016) Finding Shangri-La: limiting the impact of senescence on aging. Cell Stem Cell 18(3):305–306. https://doi.org/10.1016/j.stem.2016.02.002
Kaur IP, Kuhad A, Garg A, Chopra K (2009) Probiotics: delineation of prophylactic and therapeutic benefits. J Med Food 12(2):219–235. https://doi.org/10.1089/jmf.2007.0544
Funding
This work was supported by grants from Department of Science and Technology, Government of India, under the INSPIRE Faculty scheme (IFA17-LSPA79). The CSIR-IHBT publication number of this manuscript is 4350.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies with human or animal subjects.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, R., Sharma, A., Gupta, M. et al. Cell-Free Culture Supernatant of Probiotic Lactobacillus fermentum Protects Against H2O2-Induced Premature Senescence by Suppressing ROS-Akt-mTOR Axis in Murine Preadipocytes. Probiotics & Antimicro. Prot. 12, 563–576 (2020). https://doi.org/10.1007/s12602-019-09576-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-019-09576-z