Abstract
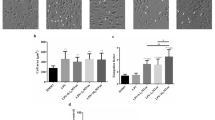
Luteolin is a natural flavonoid compound derived from vegetables, fruits, and herbs with potent anti-inflammatory activity. Macrophage polarization is important in the development and progression of inflammation. However, whether luteolin can inhibit inflammation by regulating the polarized phenotypes of macrophages remains unknown. The aim of this study was to investigate the effects of luteolin on the inflammatory polarization of macrophages and the underlying mechanisms. RAW264.7 macrophages were induced to M1 polarization by stimulation with lipopolysaccharide plus interferon-γ or to M2 polarization with interleukin 4 (IL-4), simultaneously, accompanied with different concentrations of luteolin. Laser confocal microscopy was used to observe cell morphology; flow cytometry was employed to detect the expression of membrane surface molecule CD86 and CD206; qPCR was performed to examine the mRNA expression of M1 markers (iNOS, IL-1β, IL-6) and M2 markers (Arg1, CD206, CD163, IL-10, and IL-13), respectively; ELISA was used to examine the levels of IL-6, TNF-α, and IL-10; and Western blotting was used to evaluate the p-STAT3 and p-STAT6 protein pathway. The morphology of activated M1 macrophages changed significantly, developing dendritic characteristics. After luteolin treatment, the expression of M1-type proinflammatory mediators and the surface marker CD86 were decreased evidently, but those of M2-related anti-inflammatory factors and CD206 were increased markedly. Moreover, p-STAT3 was downregulated and p-STAT6 was upregulated in a dose-dependent manner. Conclusion, luteolin can alter the M1/M2 polarization of macrophages, thereby playing an anti-inflammatory role via downregulation of p-STAT3 and upregulation of p-STAT6. Therefore, luteolin may be potentially valuable to inhibit inflammation.








Similar content being viewed by others
Abbreviations
- LPS :
-
Lipopolysaccharide
- IFN-γ :
-
Interferon-γ
- iNOS :
-
Inducible nitric oxide synthase
- IL-1β :
-
Interleukin-1β
- CD :
-
Cluster of differentiation
- TNF :
-
Tumor necrosis factor
- STAT :
-
Signal transducer and activator of transcription
References
Wang, N., H. Liang, and K. Zen. 2014. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Frontiers in Immunology 28: 614–648.
Ronco, C. 2014. Lipopolysaccharide (LPS) from the cellular wall of Gram-negative bacteria, also known as endotoxin, is a key molecule in the pathogenesis of sepsis and septic shock. Preface. Blood Purification 37: 1–1.
Kong, L.N., X. Lin, C. Huang, T. Ma, X.M. Meng, C.J. Hu, Q.Q. Wang, Y.H. Liu, Q.P. Shi, and J. Li. 2019. Hesperetin derivative-12 (HDND-12) regulates macrophage polarization by modulating JAK2/STAT3 signaling pathway. Chinese Journal of Natural Medicines 17: 0122–0130.
Perfilyeva, Y.V., N. Abdolla, Y.O. Ostapchuk, R. Tleulieva, V.C. Krasnoshtanov, A.V. Perfilyeva, and N.N. Belyaev. 2019. Chronic inflammation contributes to tumor growth: possible role of L-selectin-expressing myeloid-derived suppressor cells (MDSCs). Inflammation 42: 276–289.
Moore, K.J., and I. Tabas. 2011. Macrophages in the pathogenesis of atherosclerosis. Cell 145: 341–355.
Zhu, W., Z. Jin, J. Yu, J. Liang, Q. Yang, F. Li, X. Shi, X. Zhu, and X. Zhang. 2016. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. International Immunopharmacology 35: 119–126.
Chawla, A., K.D. Nguyen, and Y.P. Goh. 2011. Macrophage-mediated inflammation in metabolic disease. Nature Reviews Immunology 11: 738–749.
Jeong, S.K., J.S. Kim, C.G. Lee, Y.S. Park, S.D. Kim, S.O. Yoon, D.H. Han, K.Y. Lee, M.H. Jeong, and W.S. Jo. 2017. Tumor associated macrophages provide the survival resistance of tumor cells to hypoxic microenvironmental condition through IL-6 receptor-mediated signals. Immunobiology 222: 55–65.
Kapoor, N., J. Niu, Y. Saad, S. Kumar, T. Sirakova, E. Becerra, X. Li, and P.E. Kolattukudy. 2015. Transcription factors STAT6 and KLF4 implement macrophage polarization via the dual catalytic powers of MCPIP. The Journal of Immunology 194: 6011–6023.
Kiu, H., and S.E. Nicholson. 2012. Biology and significance of the JAK/STAT signalling pathways. Growth Factors 30: 88–106.
Qin, H., A.T. Holdbrooks, Y. Liu, S.L. Reynolds, L.L. Yanagisawa, and E.N. Benveniste. 2012. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. The Journal of Immunology 189: 3439–3448.
Ding, N., Y. Wang, C. Dou, F. Liu, G. Guan, K. Wei, J. Yang, M. Yang, J. Tan, and C. Zhu. 2019. Physalin D regulates macrophage M1/M2 polarization via the STAT1/6 pathway. Journal of Cellular Physiology 234: 8788–8796.
Lee, E.J., S.Y. Oh, and M.K. Sung. 2012. Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food and Chemical Toxicology 50: 4136–4143.
Leyva-López, N., E.P. Gutierrezgrijalva, D.L. Ambrizperez, and J.B. Heredia. 2016. Flavonoids as cytokine modulators: A possible therapy for inflammation-related diseases. International Journal of Molecular Sciences 17: E921.
Russo, M., I. Bonaccorsi, G. Torre, M. Sarò, P. Dugo, and L. Mondello. 2014. Underestimated sources of flavonoids, limonoids and dietary fibre: availability in lemon's by-products. Journal of Functional Foods 9: 18–26.
Wang, S., M. Cao, S. Xu, J. Zhang, Z. Wang, X. Mao, X. Yao, and C. Liu. 2017. Effect of luteolin on inflammatory responses in RAW264.7 macrophages activated with LPS and IFN-γ. Journal of Functional Foods 32: 123–130.
Feng, X., D. Weng, F. Zhou, Y.D. Owen, H. Qin, J. Zhao, W. Yu, Y. Huang, J. Chen, H. Fu, N. Yang, D. Chen, J. Li, R. Tan, and P. Shen. 2016. Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. EBioMedicine 9: 61–76.
Zhu, Y., S. Fan, Y. Lu, Y. Wei, J. Tang, Y. Yang, F. Li, Q. Chen, J. Zheng, and X. Liu. 2019. Quercetin confers protection of murine sepsis by inducing macrophage M2 polarization via the TRPM2 dependent calcium influx and AMPK/ATF3 activation. Journal of Functional Foods 56: 1–13.
O'Rourke, L., and P.R. Shepherd. 2002. Biphasic regulation of extracellular-signal-regulated protein kinase by leptin in macrophages: role in regulating STAT3 Ser727 phosphorylation and DNA binding. Biochemical Journal 364: 875–879.
Liu, H., Y. Ma, S.M. Cole, C. Zander, K.H. Chen, J. Karras, and R.M. Pope. 2003. Serine phosphorylation of STAT3 is essential for Mcl-1 expression and macrophage survival. Blood 102: 344–352.
Xia, N., G. Chen, M. Liu, X. Ye, Y. Pan, J. Ge, Y. Mao, H. Wang, J. Wang, and S. Xie. 2016. Anti-inflammatory effects of luteolin on experimental autoimmune thyroiditis in mice. Experimental and Therapeutic Medicine 12: 4049–4054.
Yu, Q., K.W. Zeng, X.L. Ma, Y. Jiang, P.F. Tu, and X.M. Wang. 2017. Ginsenoside Rk1 suppresses pro-inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells by inhibiting the Jak2/Stat3 pathway. Chinese Journal of Natural Medicines 15: 751–757.
Liu, Y.C., Y.Y. Hsiao, K.L. Ku, H.F. Liao, and W.C. Chao. 2018. Mahonia oiwakensis extract and its bioactive compounds exert anti-inflammatory activities and VEGF production through m2-macrophagic polarization and stat6 activation. Journal of Medicinal Food 21: 654–664.
Neurath, M.F., and S. Finotto. 2011. Il-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine and Growth Factor Reviews 22: 83–89.
Richard, A.C., J.R. Ferdinand, F. Meylan, E.T. Hayes, O. Gabay, and R.M. Siegel. 2015. The TNF-family cytokine TL1A: from lymphocyte costimulator to disease co-conspirator. Journal of Leukocyte Biology 98: 333–345.
Ellis, S.D., J.L. Mcgovern, A. van Maurik, D. Howe, M.R. Ehrenstein, and C.A. Notley. 2014. Induced CD8+FoxP3+Treg cells in rheumatoid arthritis are modulated by p38 phosphorylation and monocytes expressing membrane tumor necrosis factor α and CD86. Arthritis Rheumatology 66: 2694–2705.
Held, T.K., X. Weihua, L. Yuan, D.V. Kalvakolanu, and A.S. Cross. 1999. Gamma interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the, signal transduction level and via an autocrine, mechanism involving tumor necrosis factor alpha and interleukin-1. Infection and Immunity 67: 206–212.
Sodhi, A., and V. Kesherwani. 2007. Signaling molecules involved in production and regulation of IL-1beta by murine peritoneal macrophages in vitro on treatment with concanavalin a. International Immunopharmacology 7: 1403–1413.
Zhou, L., and D.R. Littman. 2009. Transcriptional regulatory networks in Th17 cell differentiation. Current Opinion in Immunology 21: 146–152.
Lau, M., E. Tsantikos, M.J. Maxwell, D.M. Tarlinton, G.P. Anderson, and M.L. Hibbs. 2012. Loss of STAT6 promotes autoimmune disease and atopy on a susceptible genetic background. Journal of Autoimmunity 39: 388–397.
Lawrence, T., and G. Natoli. 2011. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nature Reviews Immunology 11: 750–761.
Ando, Y., T. Oku, and T. Tsuji. 2016. Platelet supernatant suppresses LPS-induced nitric oxide production from macrophages accompanied by inhibition of NF-κB signaling and increased Arginase-1 expression. PLoS One 11: e0162208.
Dai, K., L. Huang, X. Sun, L. Yang, and Z. Gong. 2015. Hepatic CD206-positive macrophages express amphiregulin to promote the immunosuppressive activity of regulatory T cells in HBV infection. Journal of Leukocyte Biology 98: 1071–1080.
Etzerodt, A., and S.K. Moestrup. 2013. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxidants & Redox Signaling 18: 2352–2363.
Sica, A., and A. Mantovani. 2012. Macrophage plasticity and polarization: in vivo veritas. Journal of Clinical Investigation 122: 787–795.
Szanto, A., B.L. Balint, Z.S. Nagy, E. Barta, B. Dezso, A. Pap, L. Szeles, S. Poliska, M. Oros, R.M. Evans, Y. Barak, J. Schwabe, and L. Nagy. 2010. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity 33: 699–712.
Yuan, Q., D. Zhang, C. Liu, C. Zhang, and D. Yuan. 2018. Chikusetsusaponin V inhibits lps-activated inflammatory responses via SIRT1/NF-κB signaling pathway in RAW264.7 cells. Inflammation 41: 2149–2159.
Park, S.H., H.S. Park, J.H. Lee, G.Y. Chi, G.Y. Kim, S.K. Moon, Y.C. Chang, J.W. Hyun, W.J. Kim, and Y.H. Choi. 2013. Induction of endoplasmic reticulum stress-mediated apoptosis and non-canonical autophagy by luteolin in NCI-H460 lung carcinoma cells. Food and Chemical Toxicology 56: 100–109.
Funding
This work was supported by National Natural Science Foundation of China (grant number 81673945; 81471010) and the Project of Jiangsu Branch of China Academy of Chinese Medical Sciences (FY201809).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, S., Cao, M., Xu, S. et al. Luteolin Alters Macrophage Polarization to Inhibit Inflammation. Inflammation 43, 95–108 (2020). https://doi.org/10.1007/s10753-019-01099-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01099-7