Abstract
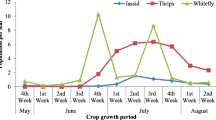
The wide spread cultivation of transgenic Bt-cotton in India has altered the pest profile from bollworms to sap-sucking insect-pests especially whitefly [Bemisia tabaci (Gennadius)]. A whitefly epidemic in 2015 destroyed cotton crop on nearly 1.5 million hectares in North Indian cotton growing states. Management of whitefly is not easy due to its wider host adaptability, potential for insecticide resistance development and rapid evolution. Host plant resistance is the most viable approach for combating whitefly menace. A wild cotton species Gossypium armourianum Kearney Accession PAU 1 tolerant to various biotic stresses was used for the introgression of whitefly tolerance in Upland cotton (Gossypium hirsutum L.). Various morpho-anatomical characteristics (viz. stomatal frequency, gossypol glands, trichome density, leaf thickness) were studied from leaf discs in experimental material (i.e. parents G. armourianum Acc. PAU 1, G. hirsutum cv. F 1861 and LH 1556, F1, BC1F1, BC2F1 derivatives). The BC2F1 individuals differed significantly in stomatal frequency, trichome density, number of gossypol glands and lamina thickness; and hence their response to whitefly tolerance. However, with age progression, a significant decline in stomatal frequency, gossypol glands and trichome density were observed in mature leaves. Correlation studies revealed a positive association of whitefly incidence with trichome density (r = 0.319*) and stomatal frequency (r = 0.372*) in young leaves. A positive correlation of stomatal frequency with gossypol gland (ry = 0.251* and rm = 0.296*) and trichome density (ry = 0.360* and rm = 0.305*) was observed in both young and mature leaves. G. armourianum possessed 4–5 -folds higher tannin contents than G. hirsutum parents before infestation. Surprisingly in G. armourianum, increase in tannin levels were observed much earlier (after 24 h of infestation) as compared to G. hirsutum.




Similar content being viewed by others
References
Abdurakhmonov, I. Y. (2013). Role of genomic studies in boosting yield. Proceedings of International Cotton Advisory Committee Board (ICAC), Cartagena, pp 7–22.
Alon, M., Elbaz, M., Ben-Zvi, M. M., Feldmesser, E., & Vainstein, A. (2012). Insights into the transcriptomics of polyphagy: Bemisia tabaci adaptability to phenyl propanoids involves coordinated expression of defense and metabolic genes. Insect Biochemistry & Molecular Biology, 42, 251–263.
Ashfaq, M., Ane, M. N., Zia, K., Nasreen, A., & Hassan, M. (2010). The correlation of abiotic factors and physico-morphic characteristics of (Bacillus thuringiensis) Bt transgenic cotton with whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) and jassid, Amrasca devastans (Homoptera: Jassidae) populations. African Journal of Agricultural Research, 5(22), 3102–3107.
Bleeker, P. M., Diergaarde, P. J., Ament, K., Guerra, J., Weidner, M., Schutz, S., de Both, M. T., Haring, M. A., & Schuurink, R. C. (2009). The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiology, 151, 925–935.
Briddon, R. W., & Markham, P. G. (2000). Cotton leaf curl virus disease. Virus Research, 71, 151–159.
Butler, G. D. J. R., Wilson, F. D., & Fishler, G. (1991). Cotton leaf trichomes and populations of Empoasca lybica and Bemisia tabaci. Crop Protection, 10, 461–464.
Cabanillas, H. E., De Leon, J. H., Humber, R. A., Murray, K. D., & Jones, W. A. (2013). Isaria poprawskii sp nov (Hypocreales: Cordycipitaceae), a new entomopathogenic fungus from Texas affecting sweet potato whitefly. Mycoscience, 54, 158–169.
Cavanagh, A., Hazzard, R., Adler, L. S., & Boucher, J. (2009). Using trap crops for control of Acalymma vittatum (Coleoptera: Chrysomelidae) reduces insecticide use in butternut squash. Journal of Economic Entomology, 102, 1101–1107.
Chavan, S. J., Bhosle, B. B., & Bhute, N. K. (2010). Estimation of losses due to major insect pests in desi cotton in Maharashtra. Journal of Cotton Research and Development, 24, 95–96.
Chen, Z. H., Chen, G., Dai, F., Wang, Y., Hills, A., & Ruan, Y. L. (2017). Molecular evolution of grass stomata. Trends in Plant Science, 22, 124–139.
De-Boer, H. J., Price, C. A., Wagner-Cremer, F., Dekker, S. C., Franks, P. J., & Veneklaas, E. J. (2016). Optimal allocation of leaf epidermal area for gas exchange. New Phytologist, 210, 1219–1228.
Dittberner, H., Korte, A., Mettler-Altmann, T., Weber, A. P. M., Monroe, G., & de Meaux, J. (2018). Natural variation in stomata size contributes to the local adaptation of water-use efficiency in Arabidopsis thaliana. Molecular Ecology. https://doi.org/10.1111/mec.14838
Doheny-Adams, T., Hunt, L., Franks, P. J., Beerling, D. J., & Gray, J. E. (2012). Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philosophical Transactions of the Royal Society London Series B Biological Science, 367, 547–555.
Drake, J. W., & Holland, J. J. (1999). Mutation rates among RNA viruses. Proceedings of the National Academy of Sciences USA, 96, 13910–13913.
Dubois, M., Gilles, K. A., Hamilton, J. K., Roberts, P. A., & Smith, F. (1956). Calorimetric methods for the determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
Fanourakis, D., Giday, H., Milla, R., Pieruschka, R., Kjaer, K. H., & Bolger, M. (2015). Pore size regulates operating stomatal conductance, while stomatal densities drive the partitioning of conductance between leaf sides. Annals of Botany, 115, 555–565.
Farooq, J., Farooq, A., Riaz, M., Shahid, M. R., Saeed, F., Hussain, M. I. T., Batool, A., & Mahmood, A. (2014). Cotton leaf curl virus disease a principle cause of decline in cotton productivity in Pakistan (a mini review). Canadian Journal of Plant Protection, 2, 9–16.
Franks, P. J., & Beerling, D. J. (2009). Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences USA, 106, 10343–10347.
García-Andrés, S., Monci, F., Navas-Castillo, J., & Moriones, E. (2006). Begomo virus genetic diversity in the native plant reservoir Solanum nigrum: Evidence for the presence of a new virus species of recombinant nature. Virology, 350, 433–442.
Goławska, S., & Łukasik, I. (2009). Acceptance of low-saponin lines of alfalfa with varied phenolic concentrations by pea aphid (Homoptera: Aphididae). Biologia, 64, 377–382.
Grover, G., Kaur, B., Pathak, D., & Kumar, V. (2016). Genetic variation for leaf trichome density and its association with sucking insect-pests incidence in Asiatic cotton. Indian Journal of Genetics, 76, 365–368.
Guo, H., Sun, Y., Peng, X., Wang, Q., Harris, M., & Ge, F. (2016). Up-regulation of abscisic acid signaling pathway facilitates aphid xylem absorption and osmoregulation under drought stress. Journal of Experimental Botany, 67(3), 681–693.
Hagg, J. F., Zagrobelny, M., & Bak, S. (2013). Plant defence against insect herbivores. International Journal of Molecular Science, 14(5), 10242–10297.
Hiscox, J. D., & Israelstam, G. F. (1979). A method for the extraction of chlorophyll from the leaf tissue without maceration. Canadian Journal of Botany, 57, 1332–1334.
Houndete, T. A., Ketoh, G. K., Hema, O. S. A., Brevault, T., Glitho, I. A., & Martin, T. (2010). Insecticide resistance in field populations of Bemisia tabaci (Hemiptera: Aleyrodidae) in West Africa. Pest Management Science, 66, 1181–1185.
India Stat. com. (2018). India Stat: Socio-economic statistical information about India. https://www.indiastat.com/
Kanher, F. M., Syed, T. S., Abro, G. H., Jahangir, T. M., & Tunio, S. A. (2016). Some physio-morphological leaf characters of gamma irradiated cotton lines to resistance against Jassid (Amrasca devastans Dist.). Journal of Entomology and Zoology Studies, 4, 80–85.
Karunker, I., Benting, J., Lueke, B., Ponge, T., Nauen, R., Roditakis, E., John Vontas, J., Gorman, K., & Denholm, I. (2008). Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochemistry & Molecular Biology, 38, 634–644.
Kaur, H., Pathak, D., & Rathore, P. (2016). Development and characterization of an interspecific Gossypium hirsutum x Gossypium armourianum hybrid. Applied Biological Research, 18, 146–154.
Keshav, A., Shera, P. S., & Singh, J. (2013). Morphological basis of resistance to spotted bollworm, Earias vittella (Fabricius) in Asiatic cotton. Phytoparasitica, 41, 235–240.
Khalil, H., Raza, A. B. M., Afzal, M., Aqueel, M. A., Khalil, M. A., & Mansoor, M. M. (2017). Effects of plant morphology on the incidence of sucking insect pests complex in few genotypes of cotton. Journal of the Saudi Society of Agricultural Sciences, 16, 344–349.
Kumar, V., Kular, J. S., Kumar, R., Sidhu, S. S., & Chhuneja, P. K. (2020). Integrated whitefly [Bemisia tabaci (Gennadius)] management in Bt-cotton in North India: An agroecosystem-wide community-based approach. Current Science, 119, 618–624.
Li, S. J., Xue, X., Ahmed, M. Z., Ren, S. X., & Du, Y. Z. (2011). Host plants and natural enemies of Bemisia tabaci (Hemiptera: Aleyrodidae) in China. Journal of Insect Science, 18, 101–120.
Lowry, O. H., Rosenbrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Lu, Y. H., Wu, K. M., Wyckhuys, K. A. G., & Guo, Y. Y. (2009). Potential of mungbean, Vigna radiatus as a trap crop for managing Apolygus lucorum (Hemiptera: Miridae) on Bt cotton. Crop Protection, 28, 77–81.
Luo, C., Jones, C. M., Devine, G., Zhang, F., Denholm, I., & Gorman, K. (2010). Insecticides resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China. Crop Protection, 29, 429–434.
Mansour, M. H., Zohdy, N. M., El-Gengaihi, S. E., & Amr, A. E. (1997). The relationship between tannins concentration in some cotton varieties and susceptibility to piercing sucking insects. Journal of Applied Entomology, 121, 321–325.
Miyazaki, J., Stiller, W. N., & Wilson, L. J. (2013). Identification of host plant resistance to silver leaf whitefly in cotton: Implications for breeding. Field Crops Research, 154, 145–152.
Monga, D., Narula, A. M., & Raj, S. (2001). Management of cotton leaf curl virus- A dreaded disease in North India. Proceedings of National seminar on sustainable cotton production to meet the future requirement of industry, Directorate of Cotton Development, Government of India, pp 112–15.
Muller, H. M., Schafer, N., Bauer, H., Geiger, D., Lautner, S., Fromm, J., et al. (2017). The desert plant Phoenix dactylifera closes stomata via nitrate-regulated SLAC1 anion channel. New Phytologist, 216, 150–162.
Murugesan, N., & Kavitha, A. (2010). Host plant resistance in cotton accessions to the leafhopper Amrasca devastans (Distant). Journal of Biopesticides, 3, 526–533.
Mwila, N., Rubaihayo, P. R., Kyamanywa, S., Odong, T. L., Nuwamanya, E., Mwala, M., Agbahoungba, S., & Badji, A. (2017). Biochemical factors associated with cassava resistance to whitefly infestation. African Crop Science Journal, 25(3), 365–385.
Naranjo, S. E., & Ellsworth, P. C. (2017). Whitefly IPM in cotton: Ecological, biological and socio-economic components contributing to success. Indo-US symposium on curbing whitefly-plant virus pandemics- The departure from pesticides to genomics solutions, Punjab Agricultural University, Ludhiana, India, pp 23–24.
Naveen, N. C., Chaubey, R., Kumar, D., Rebijith, K. B., Rajagopal, R., Subrahmanyam, B., & Subramanian, S. (2017). Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Scientific Reports, 7, 40634.
Nawab, N. N., Khan, I. A., Khan, A. A., & Amjad, M. (2011). Characterization and inheritance of cotton leaf pubescence. Pakistan Journal of Botany, 43, 649–658.
Pathak, D., Bala, S., Rathore, P., Sekhon, P. S., & Singh, K. (2016a) Identification of new sources of resistance to cotton leaf curl disease and its introgression in American cotton. Proceedings of the 6th World Cotton Research Conference, Goiania, Brazil, pp 50–51.
Pathak, D., Kaur, H., Rathore, P., Singh, B., Bala, S., Sekhon, P. S. & Singh, K. (2016b). Introgression of cotton leaf curl disease resistance from Gossypium armourianum into G. hirsutum. Proceedings of 1st International Agrobiodiversity Congress, New Delhi, pp 198.
Phulse, V. B., & Udikeri, S. S. (2018). Tracking in season dynamics of insecticide resistance in aphids Aphis gossypii (Glover) in different cotton growing ecosystems. Journal of Cotton Research & Development, 32, 276–283.
Pushpam, R., & Raveendran, T. S. (2006). Production of interspecific hybrids between Gossypium hirsutum and jassid resistant wild species G. raimondii and G. armourianum. Cytologia, 71, 407–418.
Qi, X., & Torii, K. U. (2018). Hormonal and environmental signals guiding stomatal development. BMC Biology, 16, 21.
Rathore, K. S. (2017). Transgenic cotton for trait improvement and applications to plant virus and whitefly resistance. Indo-US symposium on curbing whitefly-plant virus pandemics-The departure from pesticides to genomics solutions, Punjab Agricultural University, Ludhiana, India, pp 38–40.
Razaq, M., Suhail, A., Aslam, M., Arif, M. J., Saleem, M. A., & Khan, H. A. (2013). Patterns of insecticides used on cotton before introduction of genetically modified cotton in Southern Punjab, Pakistan. Pakistan Journal of Zoology, 45, 574–577.
Schuster, D. J., Mann, R. S., Toapanta, M., Cordero, R., Thompson, S., & Cyman, S. (2010). Monitoring neonicotinoid resistance in biotype B of Bemisia tabaci in Florida. Pest Management Science, 66, 186–195.
Shakeel, A., Farooq, J., Ali, M. A., Riaz, M., Farooq, A., Saeed, A., & Saleem, M. F. (2011). Inheritance pattern of earliness in cotton (Gossypium hirsutum L.). Australian Journal of Crop Science, 5, 1224–1231.
Singh, B. (2018). Introgression of cotton leaf curl disease resistance from Gossypium armourianum to G. hirsutum. M.Sc. Thesis, Punjab Agricultural University, Ludhiana.
Singh, B., Pathak, D., & RathorePooja, P. (2019). Segregation distortion in cotton. Agricultural Research Journal, 56(1), 13–16.
Sulistyo, A., & Inayati, A. (2016). Mechanisms of antixenosis, antibiosis, and tolerance of fourteen soybean genotypes in response to whiteflies (Bemisia tabaci). Biodiversitas, 17(2), 447–453.
Sun, Y., Yan, F., Cui, X., & Liu, F. (2014). Plasticity in stomatal size and density of potato leaves under different irrigation and phosphorus regimes. Journal of Plant Physiology, 171, 1248–1255.
Sun, Y., Guo, H., Yuan, L., Wei, J., Zhang, W., & Ge, F. (2015). Plant stomatal closure improves aphid feeding under elevated CO2. Global Change Biology, 21, 2739–2748.
Swain, T., & Hills, W. E. (1959). The phenolic constituents of Prunus domestica. I- The quantitative analysis of phenolic constituents. Journal of the Science of Food and Agriculture, 10, 63–68.
Taggar, G. K., & Gill, R. S. (2012). Preference of whitefly, Bemisia tabaci, towards black gram genotypes: Role of morphological leaf characteristics. Phytoparasitica, 40, 461–474.
Taggar, G. K., Gill, R. S., Gupta, A. K., & Singh, S. (2014). Induced changes in the antioxidative compounds of Vigna mungo genotypes due to infestation by Bemisia tabaci (Gennadius). Journal of Environmental Biology, 35(6), 1037–1045.
Tezcan, F., Goven, M. A., Demir, G., & Topuz, M. (2000). Pamukta Entegre Mücadele Uretici Broşsuru. Bornova Zirai Mucadele Araştırma Enstitusu, yayın no: 2002/5, 32 s, Gzmir.
Trapero, C., Wilson, I. W., Stiller, W. N., & Wilson, L. J. (2016). Enhancing integrated pest management in GM cotton systems using host plant resistance. Frontiers in Plant Science, 7, 500.
Vyskocilova, S., Tay, W. T., Brunschot, S. V., Susan, S., & Colvin, J. (2018). An integrative approach to discovering cryptic species within the Bemisia tabaci whitefly species complex. Scientific Reports, 8, 10886.
Wagner, G., Wang, E., & Shepherd, R. (2004). New approaches for studying and exploiting an old protuberance, the plant trichome. Annals of Botany, 93, 3–11.
Wang, Z. Y., Yao, M. D., & Wu, Y. D. (2009). Cross-resistance, inheritance and biochemical mechanisms of imidacloprid resistance in B-biotype Bemisia tabaci. Pest Management Science, 65, 1189–1194.
War, A. R., Paulraj, M. G., Ahmad, T., Buhroo, A. A., Hussain, B., Ignacimuthu, S., & Sharma, H. C. (2012). Mechanisms of plant defence against insect herbivores. Plant Signalling & Behaviour, 7(10), 1306–1320.
Wendel, J. F., Flagel, L., & Adams, K. L. (2012). Jeans, genes, and genomes: Cotton as a model for studying polyploidy. In P. S. Soltis & D. E. Soltis (Eds.), Polyploidy and genome evolution (pp. 181–208). Springer.
Werker, E. (2000). Trichome diversity and development. In D. L. Hallahan & J. C. Gray (Eds.), Advances in botanical research (pp. 1–35). Elsevier Science.
Wright, R. J., Thaxton, P. M., El-Zik, K. M., & Paterson, A. H. (1999). Molecular mapping of genes affecting pubescence of cotton. Journal of Heredity, 90(1), 215–219.
Zia, K., Ashfaq, M., Arif, M. J., & Sahi, S. T. (2011). Effect of physico-morphic characters on population of whitefly Bemisia tabaci in transgenic cotton. Pakistan Journal of Agricultural Sciences, 48(1), 63–69.
Zaidi, S. S. E. A., Naqvi, R. Z., Asif, M., Strickler, S., Shakir, S., Shafiq, M., Khan, A. M., Amin, I., Mishra, B., Mukhtar, M. S., & Scheffler, B. E. (2020). Molecular insight into cotton leaf curl geminivirus disease resistance in cultivated cotton (Gossypium hirsutum). Plant Biotechnology Journal, 18(3), 691–706.
Funding
The plant material was developed under the Programme Support on “Enhancing Durability of Resistance to Biotic Stresses in Selected Cereal and Fiber Crops through Biotechnological Approaches (BT/01/CE1B/121/01)” funded by the Department of Biotechnology, Government of India. Ministry of Science and Technology (grant no. 102/IFD/SAN/1307/2014-15).
Author information
Authors and Affiliations
Contributions
N. Gupta and D. Pathak designed research. D. Pathak and P. Rathore developed and maintained the germplasm. T. Suthar and N. Gupta recorded the morphoanatomical observations and performed the statistical analysis. T. Suthar and S. Sharma conducted biochemical profiling. N. Gupta and D. Pathak interpreted the data and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suthar, T., Gupta, N., Pathak, D. et al. Morpho-anatomical characterization of interspecific derivatives of Gossypium hirsutum L. × G. armourianum Kearney cross for whitefly tolerance. Phytoparasitica 50, 423–441 (2022). https://doi.org/10.1007/s12600-021-00963-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-021-00963-3