Abstract
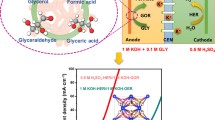
Energy-saving glycerol electrolysis with lower potential than water spitting endows a promising way for the concurrent production of value-added formate and high-purity hydrogen. However, there is still lack of efficient electrocatalysts at both anode and cathode for glycerol electrolysis. Herein, we report the activation of Ni site in NiV layered double hydroxide (LDH) by electrochemical and N2/H2 plasma regulations for boosting the activity of glycerol oxidation reaction (GOR) and hydrogen evolution reaction (HER), respectively. Specifically, boosted GOR performance with a low overpotential (1.23 V at 10 mA·cm−2) and a high Faradic efficiency (94%) is demonstrated by electrochemically regulated NiV LDH (E-NiV LDH) with elevated valence state of Ni site. In situ Raman spectrum reveals the generation of Ni(III) species by electrochemical regulation, and the highly active Ni(III) can be regenerated with the process of electrochemical oxidation. Additionally, the possible reaction pathway is speculated based on the in situ Fourier transform infrared spectroscopy (FTIR) and high-performance liquid chromatography results. The plasma-regulated NiV LDH (P-NiV LDH) with lower valence state of Ni site exhibits outstanding HER activity, displaying a low overpotential of 45 mV to deliver 10 mA·cm−2. When employing E-NiV LDH and P-NiV LDH as anode and cathode electrocatalyst, respectively, the assembled electrolyzer merely needs 1.25 V to achieve 10 mA·cm−2 for simultaneous production of formate and hydrogen, demonstrating remarkable 320 mV of lower potential than water electrolysis.
Graphic abstract

摘要
电解甘油比电解水具有更低的电压‚ 为同时生产高附加值的甲酸和高纯氢气提供了一种有前景的方法。然而‚ 甘油电解的阳极和阴极均缺乏高效的电催化剂。本文报道了电化学和N2/H2等离子体调控NiV LDH 中的Ni位点分别提高甘油氧化反应(GOR)和析氢反应(HER)的活性。经电化学调控的、具有高价镍物种的NiV LDH (E-NiV LDH) 展现出增强的GOR性能‚ 表现出低的过电位(10 mA·cm−2仅需1.23 V)和高的法拉第效率(94%)。原位拉曼光谱揭示了电化学调控过程中Ni(III)物种的生成‚ 并通过电化学氧化过程实现了高活性Ni(III)的再生。此外‚ 根据原位FTIR和高效液相色谱的结果推测了可能的甘油氧化反应途径。经等离子体调控的、含低价镍物种的NiV LDH (P-NiV LDH)表现出优异的HER活性‚ 其实现10 mA·cm−2的电流密度仅需45 mV的低过电位。当E-NiV LDH和P-NiV LDH分别作为阳极和阴极电催化剂时‚ 组装成的电解槽只需1.25 V即可达到10 mA·cm−2电流密度‚ 同时产生甲酸和氢气‚ 比电解水所需的电位低320 mV。






Similar content being viewed by others
References
Gao R, Yan D. Recent development of Ni/Fe-based micro/nanostructures toward photo/electrochemical water oxidation. Adv Energy Mater. 2019;10(11):1900954.
Zhao G, Rui K, Dou SX, Sun W. Heterostructures for electrochemical hydrogen evolution reaction: a review. Adv Funct Mater. 2018;28(43):1803291.
Zhang JY, Yan Y, Mei B, Qi R, He T, Wang Z, Fang W, Zaman S, Su Y, Ding S, Xia BY. Local spin-state tuning of cobalt–iron selenide nanoframes for the boosted oxygen evolution. Energy Environ Sci. 2021;14(1):365.
Lu YX, Dong CL, Huang YC, Zou Y, Liu Z, Liu Y, Li Y, He N, Shi JQ, Wang SY. Identifying the geometric site dependence of spinel oxides for the electrooxidation of 5-hydroxymethylfurfural. Angew Chem Int Ed. 2020;59(43):19215.
Jenny ST, Guo YJ, Yu HZ. Interactions between hemin-binding DNA aptamers and hemin–graphene nanosheets: reduced affinity but unperturbed catalytic activity. J Anal Test. 2019;3(1):107.
Li M, Deng X, Xiang K, Liang Y, Zhao B, Hao J, Luo JL, Fu XZ. Value-added formate production from selective methanol oxidation as anodic reaction to enhance electrochemical hydrogen cogeneration. Chemsuschem. 2020;13(5):914.
Liu S, Wang X, Yu HG, Wu YP, Li B, Lan YQ, Wu T, Zhang J, Li DS. Two new pseudo-isomeric nickel (II) metal–organic frameworks with efficient electrocatalytic activity toward methanol oxidation. Rare Met. 2021;40(2):489.
Zheng D, Li J, Ci S, Cai P, Ding Y, Zhang M, Wen Z. Three-birds-with-one-stone electrolysis for energy-efficiency production of gluconate and hydrogen. Appl Catal B Environ. 2020;277:119178.
Yang Y, Mu T. Electrochemical oxidation of biomass derived 5-hydroxymethylfurfural (HMF): pathway, mechanism, catalysts and coupling reactions. Green Chem. 2021;23(12):4228.
Liu WJ, Xu Z, Zhao D, Pan XQ, Li HC, Hu X, Fan ZY, Wang WK, Zhao GH, Jin S, Huber GW, Yu HQ. Efficient electrochemical production of glucaric acid and H2 via glucose electrolysis. Nat Commun. 2020;11:265.
Li Y, Wei X, Chen L, Shi J, He M. Nickel-molybdenum nitride nanoplate electrocatalysts for concurrent electrolytic hydrogen and formate productions. Nat Commun. 2019;10:5355.
Esmaeili A, Kirk DW. Water removal in the alkaline electrochemical valorization of glycerol by pervaporation. Sep Purif Technol. 2020;248:116943.
Houache MSE, Safari R, Nwabara UO, Rafaïdeen T, Botton GA, Kenis PJA, Baranton S, Coutanceau C, Baranova EA. Selective electrooxidation of glycerol to formic acid over carbon supported Ni1–xMx (M = Bi, Pd, and Au) nanocatalysts and coelectrolysis of CO2. ACS Appl Energy Mater. 2020;3(9):8725.
Liu C, Hirohara M, Maekawa T, Chang R, Hayashi T, Chiang CY. Selective electro-oxidation of glycerol to dihydroxyacetone by a non-precious electrocatalyst—CuO. Appl Catal B Environ. 2020;265:118543.
Han X, Sheng H, Yu C, Walker TW, Huber GW, Qiu J, Jin S. Electrocatalytic oxidation of glycerol to formic acid by CuCo2O4 spinel oxide nanostructure catalysts. ACS Catal. 2020;10(12):6741.
Dodekatos G, Schünemann S, Tüysüz H. Recent advances in thermo-, photo-, and electrocatalytic glycerol oxidation. ACS Catal. 2018;8(7):6301.
Dong C, Yuan X, Wang X, Liu X, Dong W, Wang R, Duan Y, Huang F. Rational design of cobalt–chromium layered double hydroxide as a highly efficient electrocatalyst for water oxidation. J Mater Chem A. 2016;4(29):11292.
Deng X, Huang J, Wan H, Chen F, Lin Y, Xu X, Ma R, Sasaki T. Recent progress in functionalized layered double hydroxides and their application in efficient electrocatalytic water oxidation. J Energy Chem. 2019;32:93.
Liu WJ, Dang L, Xu Z, Yu HQ, Jin S, Huber GW. Electrochemical oxidation of 5-hydroxymethylfurfural with NiFe layered double hydroxide (LDH) nanosheet catalysts. ACS Catal. 2018;8(6):5533.
Bender MT, Lam YC, Hammes-Schiffer S, Choi KS. Unraveling two pathways for electrochemical alcohol and aldehyde oxidation on NiOOH. J Am Chem Soc. 2020;142(51):21538.
Taitt BJ, Nam DH, Choi KS. A comparative study of nickel, cobalt, and iron oxyhydroxide anodes for the electrochemical oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. ACS Catal. 2018;9(1):660.
Wang K, Huang W, Cao Q, Zhao Y, Sun X, Ding R, Lin W, Liu E, Gao P. Engineering NiF3/Ni2P heterojunction as efficient electrocatalysts for urea oxidation and splitting. Chem Eng J. 2022;427:130865.
Dang L, Liang H, Zhuo J, Lamb BK, Sheng H, Yang Y, Jin S. Direct synthesis and anion exchange of noncarbonate-intercalated NiFe-layered double hydroxides and the influence on electrocatalysis. Chem Mater. 2018;30(13):4321.
Wang Q, O’Hare D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev. 2012;112(7):4124.
Mccrory CC, Jung S, Ferrer IM, Chatman SM, Peters JC, Jaramillo TF. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J Am Chem Soc. 2015;137(13):4347.
Zheng Y, Jiao Y, Vasileff A, Qiao SZ. The hydrogen evolution reaction in alkaline solution: from theory, single crystal models, to practical electrocatalysts. Angew Chem Int Ed. 2018;57(26):7568.
Chen QQ, Hou CC, Wang CJ, Yang X, Shi R, Chen Y. Ir(4+)-doped NiFe LDH to expedite hydrogen evolution kinetics as a Pt-like electrocatalyst for water splitting. Chem Commun. 2018;54(49):6400.
Cai Z, Wu A, Yan H, Tian C, Guo D, Fu H. Zn-doped porous CoNiP nanosheet arrays as efficient and stable bifunctional electrocatalysts for overall water splitting. Energy Technol. 2019;8(1):1901079.
Zhang P, Lu XF, Nai J, Zang SQ, Lou XW. Construction of hierarchical Co-Fe oxyphosphide microtubes for electrocatalytic overall water splitting. Adv Sci. 2019;6(17):1900576.
Anantharaj S, Sugime H, Noda S. Surface amorphized nickel hydroxy sulphide for efficient hydrogen evolution reaction in alkaline medium. Chem Eng J. 2021;408:127275.
Tong R, Sun Z, Zhang F, Wang X, Xu J, Shi X, Wang S, Pan H. N and V coincorporated Ni nanosheets for enhanced hydrogen evolution reaction. ACS Sustain Chem Eng. 2018;6(12):16525.
Zhang XY, Yuan H, Mao F, Wen CF, Zheng LR, Liu PF, Yang HG. Boosting alkaline hydrogen evolution electrocatalysis over metallic nickel sites through synergistic coupling with vanadium sesquioxide. Chemsuschem. 2019;12(23):5063.
Liu B, He B, Peng HQ, Zhao Y, Cheng J, Xia J, Shen J, Ng TW, Meng X, Lee CS, Zhang W. Unconventional nickel nitride enriched with nitrogen vacancies as a high-efficiency electrocatalyst for hydrogen evolution. Adv Sci. 2018;5(8):1800406.
Hua W, Sun HH, Xu F, Wang JG. A review and perspective on molybdenum-based electrocatalysts for hydrogen evolution reaction. Rare Met. 2020;39:335.
Yang W, Chen S. Recent progress in electrode fabrication for electrocatalytic hydrogen evolution reaction: a mini review. Chem Eng J. 2002;393:124726.
Dong X, Yan H, Jiao Y, Guo D, Wu A, Yang G, Shi X, Tian C, Fu H. 3D hierarchical V-Ni-based nitride heterostructure as a highly efficient pH-universal electrocatalyst for the hydrogen evolution reaction. J Mater Chem A. 2019;7(26):15823.
Silversmit G, Depla D, Poelman H, Marin GB, De Gryse R. Determination of the V 2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J Electron Spectrosc Relat Phenom. 2004;135(2–3):167.
Li P, Duan X, Kuang Y, Li Y, Zhang G, Liu W, Sun X. Tuning electronic structure of NiFe layered double hydroxides with vanadium doping toward high efficient electrocatalytic water oxidation. Adv Energy Mater. 2018;8(15):1703341.
Selvam NCS, Du L, Xia BY, Yoo PJ, You B. Reconstructed water oxidation electrocatalysts: the impact of surface dynamics on intrinsic activities. Adv Funct Mater. 2020;31(12):2008190.
Yeo BS, Bell AT. In situ Raman study of nickel oxide and gold-supported nickel oxide catalysts for the electrochemical evolution of oxygen. J Phys Chem C. 2012;116(15):8394.
Yu J, Cao Q, Li YB, Xia L, Yang SH, Clark JK, Nakabayashi M, Shibata N, Delaunay JJ. Defect-rich NiCeOx electrocatalyst with ultrahigh stability and low overpotential for water oxidation. ACS Catal. 2019;9(2):1605.
Meng C, Lin MC, Sun XC, Chen XD, Chen XM, Du XW, Zhou Y. Laser synthesis of oxygen vacancy-modified CoOOH for highly efficient oxygen evolution. Chem Commun. 2019;55(20):2904.
Harley P, Rajamathi M. Cannizzaro reactions over calcined hydrotalcite. Appl Clay Sci. 2019;174:86.
Li J, Wei R, Wang X, Zuo Y, Han X, Arbiol J, Llorca J, Yang Y, Cabot A, Cui C. Selective methanol-to-formate electrocatalytic conversion on branched nickel carbide. Angew Chem Int Ed. 2020;59(47):20826.
Collins SE, Baltanás MA, Bonivardi AL. Mechanism of the decomposition of adsorbed methanol over a Pd/α, β-Ga2O3 catalyst. Appl Catal A Gen. 2005;295(2):126.
Zhang P, Sheng X, Chen X, Fang Z, Jiang J, Wang M, Li F, Fan L, Ren Y, Zhang B, Timmer BJJ, Ahlquist MSG, Sun L. Paired electrocatalytic oxygenation and hydrogenation of organic substrates with water as the oxygen and hydrogen source. Angew Chem Int Ed. 2019;58(27):9155.
Huang H, Yu C, Han X, Huang H, Wei Q, Guo W, Wang Z, Qiu J. Ni, Co hydroxide triggers electrocatalytic production of high-purity benzoic acid over 400 mA·cm−2. Energy Environ Sci. 2020;13(12):4990.
Li G, Wu X, Guo H, Guo Y, Chen H, Wu Y, Zheng J, Li X. Plasma transforming Ni(OH)2 nanosheets into porous nickel nitride sheets for alkaline hydrogen evolution. ACS Appl Mater Interfaces. 2020;12(5):5951.
Guo Y, Zhang C, Wu Y, Yu H, Zhang S, Du A, Ostrikov K, Zheng J, Li X. Direct conversion of metal organic frameworks into ultrafine phosphide nanocomposites in multicomponent plasma for wide ph hydrogen evolution. J Mater Chem A. 2020;8(20):10402.
Wang J, Xie Y, Yao Y, Huang X, Willinger M, Shao L. Ni/NiO nanoparticles on a phosphorous oxide/graphene hybrid for efficient electrocatalytic water splitting. J Mater Chem A. 2017;5(28):14758.
Zhang T, Wu MY, Yan DY, Mao J, Liu H, Hu WB, Du XW, Ling T, Qiao SZ. Engineering oxygen vacancy on NiO nanorod arrays for alkaline hydrogen evolution. Nano Energy. 2018;43:103.
Li J, Yao C, Kong X, Li Z, Jiang M, Zhang F, Lei X. Boosting hydrogen production by electrooxidation of urea over 3D hierarchical Ni4N/Cu3N nanotube arrays. ACS Sustain Chem Eng. 2019;7(15):13278.
Jin Z, Wei T, Li F, Zhang Q, Xu L. Fabrication of a novel Ni3N/Ni4N heterojunction as a non-noble metal co-catalyst to boost the H2 evolution efficiency of Zn0.5Cd0.5S. New J Chem. 2020;44(8):3471.
Wan LJ, Hu JS, Lu G, Gu L, Huang LB, Zhang QH, Zhao Z, Zhang Y, Zhao L. Steering elementary steps towards efficient alkaline hydrogen evolution via size-dependent Ni/NiO nanoscale heterosurfaces. Natl Sci Rev. 2020;7(1):27.
Acknowledgements
This study was financially supported by the National Science Foundation of China (No. 12075002), the Outstanding Youth Fund of Anhui Province (No. 2008085J21), Anhui Provincial Supporting Program for Excellent Young Talents in Universities (No. gxyqZD2019005), and the Innovation and Entrepreneurship Project of Overseas Returnees in Anhui Province (No. 2019LCX018).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, L., Chang, GR., Feng, Y. et al. Regulating Ni site in NiV LDH for efficient electrocatalytic production of formate and hydrogen by glycerol electrolysis. Rare Met. 41, 1583–1594 (2022). https://doi.org/10.1007/s12598-021-01881-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01881-3