Abstract
As the most hazardous side reaction, Li plating poses high risks of undermining electrochemical performance of Li-ion batteries by accelerating degradation. Under some extreme abuse conditions, Li plating can even jeopardize safety performance and induce catastrophic results like thermal runaway. Therefore, multiscale observation of Li plating is of great significance for understanding the internal mechanisms and early detection of Li plating. In this mini-review, the recent progress of formation mechanisms of plated metallic lithium was introduced. Then, the in situ and ex situ observation methods of macroscopic, microscopic and atomic level were summarized. Reference electrode provides a promising tool for real-time monitoring of anode potential, which is the critical factor of Li plating, showing great potentials in cloud-based battery management systems. Finally, some perspectives for future researches on Li plating observation and corresponding utilizations in developing Li plating free control strategies were proposed.

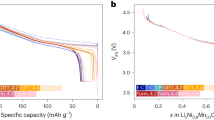
Reproduced with permission from Ref. [33]. Copyright 2019, IOP. c Differential voltage and d differential capacity curves during discharging process. Reproduced with permission from Ref. [48]. Copyright 2014, Elsevier. A summary of e fade, f coulombic efficiency and g coulombic inefficiency per hour (CIE/h) versus charge rate for all pouch cells at different temperatures and rates using both single and two-stage charge process. Reproduced with permission from Ref. [19]. Copyright 2015, IOP

Reproduced with permission from Ref. [17]. Copyright 2019, Royal Society of Chemistry. d, e SEM images of a graphite anode during charging process without Li plating; f, g an anode surface with Li plating induced by 10C pulse charging, which was quickly disassembled within less than 5 min; h, i an anode after 10C pulse charging followed by prolonged relaxation. Reproduced with permission from Ref. [16]. Copyright 2015, Elsevier

Reproduced with permission from Ref. [60]. Copyright 2014, Elsevier. d Raw data of diffraction reflections of LiC12 and LiC6 for the lowest charging rate C/20 and the highest charging rate 1C directly after charging and after a 4-h rest. Reproduced with permission from Ref. [61]. Copyright 2017, Elsevier
Similar content being viewed by others
References
Tarascon JM, Armand M. Issues and challenges facing rechargeable lithium batteries. Nature. 2001;414(6861):359.
Zubi G, Dufo-López R, Carvalho M, Pasaoglu G. The lithium-ion battery: state of the art and future perspectives. Renew Sustain Energy Rev. 2018;89:292.
Scrosati B, Hassoun J, Sun YK. Lithium-ion batteries. A look into the future. Energy Environ Sci. 2011;4(9):3287.
Feng X, Ren D, He X, Ouyang M. Mitigating thermal runaway of lithium-ion batteries. Joule. 2020;4(4):743.
Wu B, Widanage WD, Yang S, Liu X. Battery digital twins: perspectives on the fusion of models, data and artificial intelligence for smart battery management systems. Energy AI. 2020;1:100016.
Yang S, He R, Zhang Z, Cao Y, Gao X, Liu X. Cyber hierarchy and interactive network (CHAIN)––enabling digital solution for battery full-lifespan management. Matter. 2020;3(1):1.
Waldmann T, Hogg BI, Wohlfahrt-Mehrens M. Li plating as unwanted side reaction in commercial Li-ion cells—a review. J Power Sources. 2018;384:107.
Liu Q, Du C, Shen B, Zuo P, Cheng X, Ma Y, Yin G, Gao Y. Understanding undesirable anode lithium plating issues in lithium-ion batteries. RSC Adv. 2016;6(91):88683.
Meng X, Xu Y, Cao H, Lin X, Ning P, Zhang Y, Garcia Y, Sun Z. Internal failure of anode materials for lithium batteries—a critical review. Green Energy Environ. 2020;5(1):22.
Li T, Liu H, Shi P, Zhang Q. Recent progress in carbon/lithium metal composite anode for safe lithium metal batteries. Rare Met. 2018;37(6):449.
Pu KC, Zhang X, Qu XL, Hu JJ, Li HW, Gao MX, Pan HG, Liu YF. Recently developed strategies to restrain dendrite growth of Li metal anodes for rechargeable batteries. Rare Met. 2020;39(6):616.
Wei WQ, Liu BQ, Gan YQ, Ma HJ, Cui DW. Protecting lithium metal anode in all-solid-state batteries with a composite electrolyte. Rare Met. 2021;40(2):409.
Ren D, Hsu H, Li R, Feng X, Guo D, Han X, Lu L, He X, Gao S, Hou J, Li Y, Wang Y, Ouyang M. A comparative investigation of aging effects on thermal runaway behavior of lithium-ion batteries. eTransportation. 2019;2:100034.
Li Y, Feng X, Ren D, Ouyang M, Lu L, Han X. Thermal runaway triggered by plated lithium on the anode after fast charging. ACS Appl Mater Interfaces. 2019;11(50):46839.
Xie W, Liu X, He R, Li Y, Gao X, Li X, Peng Z, Feng S, Feng X, Yang S. Challenges and opportunities toward fast-charging of lithium-ion batteries. J Energy Storage. 2020;32:101837.
Uhlmann C, Illig J, Ender M, Schuster R, Ivers-Tiffée E. In situ detection of lithium metal plating on graphite in experimental cells. J Power Sources. 2015;279:428.
Rangarajan SP, Barsukov Y, Mukherjee PP. In operando signature and quantification of lithium plating. J Mater Chem A. 2019;7(36):20683.
Ren D, Smith K, Guo D, Han X, Feng X, Lu L, Ouyang M, Li J. Investigation of lithium plating-stripping process in Li-ion batteries at low temperature using an electrochemical model. J Electrochem Soc. 2018;165(10):A2167.
Burns JC, Stevens DA, Dahn JR. In-situ detection of lithium plating using high precision coulometry. J Electrochem Soc. 2015;162(6):A959.
Yang XG, Liu T, Gao Y, Ge S, Leng Y, Wang D, Wang C. Asymmetric temperature modulation for extreme fast charging of lithium-ion batteries. Joule. 2019;3(12):P3002.
Wang H, Zhu Y, Kim SC, Pei A, Li Y, Boyle DT, Wang H, Zhang Z, Ye Y, Huang W, Liu Y, Xu J, Li J, Liu F, Cui Y. Underpotential lithium plating on graphite anodes caused by temperature heterogeneity. Proc Natl Acad Sci U S A. 2020;117(47):29453.
Fear C, Parmananda M, Kabra V, Carter R, Love CT, Mukherjee PP. Mechanistic underpinnings of thermal gradient induced inhomogeneity in lithium plating. Energy Storage Mater. 2021;35:500.
Angeles Cabañero M, Altmann J, Gold L, Boaretto N, Müller J, Hein S, Zausch J, Kallo J, Latz A. Investigation of the temperature dependence of lithium plating onset conditions in commercial Li-ion batteries. Energy. 2019;171:1217.
Cheng XB, Zhang R, Zhao CZ, Zhang Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem Rev. 2017;117(15):10403.
Wang H, He J, Liu J, Qi S, Wu M, Wen J, Chen Y, Feng Y, Ma J. Electrolytes enriched by crown ethers for lithium metal batteries. Adv Funct Mater. 2020;31(2):2002578.
Qi S, Wang H, He J, Liu J, Cui C, Wu M, Li F, Feng Y, Ma J. Electrolytes enriched by potassium perfluorinated sulfonates for lithium metal batteries. Sci Bull. 2020. https://doi.org/10.1016/j.scib.2020.09.018.
Jiao C, Sun HB, Zhang L, Zhao SQ, Pang GY, Zhao CR, Lu SG. A high-performance lithium anode based on N-doped composite graphene. Rare Met. 2019. https://doi.org/10.1007/s12598-019-01263-w.
Gao X, Zhou YN, Han D, Zhou J, Zhou D, Tang W, Goodenough J. Thermodynamic understanding of Li-dendrite formation. Joule. 2020;4(9):1864.
Ma D, Cao Z, Hu A. Si-based anode materials for Li-ion batteries: a mini review. Nano-Micro Lett. 2014;6(4):347.
Luo F, Liu B, Zheng J, Chu G, Zhong K, Li H, Huang X, Chen L. Review—nano-silicon/carbon composite anode materials towards practical application for next generation Li-ion batteries. J Electrochem Soc. 2015;162(14):A2509.
Zuo X, Zhu J, Müller-Buschbaum P, Cheng YJ. Silicon based lithium-ion battery anodes: a chronicle perspective review. Nano Energy. 2017;31:113.
Li X, Colclasure AM, Finegan DP, Ren D, Shi Y, Feng X, Cao L, Yang Y, Smith K. Degradation mechanisms of high capacity 18650 cells containing Si-graphite anode and nickel-rich NMC cathode. Electrochim Acta. 2019;297:1109.
Rodrigues MTF, Kalaga K, Trask SE, Dees DW, Shkrob IA, Abraham DP. Fast charging of Li-ion cells: part I. Using Li/Cu reference electrodes to probe individual electrode potentials. J Electrochem Soc. 2019;166(6):A996.
Rieger B, Schuster SF, Erhard SV, Osswald PJ, Rheinfeld A, Willmann C, Jossen A. Multi-directional laser scanning as innovative method to detect local cell damage during fast charging of lithium-ion cells. J Energy Storage. 2016;8:1.
Bitzer B, Gruhle A. A new method for detecting lithium plating by measuring the cell thickness. J Power Sources. 2014;262:297.
Rangarajan SP, Barsukov Y, Mukherjee P. Anode potential controlled charging prevents lithium plating. J Mater Chem A. 2020;8:13077.
Zhou J, Notten PHL. Development of reliable lithium microreference electrodes for long-term in situ studies of lithium-based battery systems. J Electrochem Soc. 2004;151(12):A2173.
Solchenbach S, Pritzl D, Kong EJY, Landesfeind J, Gasteiger HA. A gold micro-reference electrode for impedance and potential measurements in lithium ion batteries. J Electrochem Soc. 2016;163(10):A2265.
Hou J, Girod R, Nianias N, Shen TH, Fan J, Tileli V. Lithium-gold reference electrode for potential stability during in situ electron microscopy studies of lithium-ion batteries. J Electrochem Soc. 2020;167(11):110515.
Yi S, Wang B, Chen Z, Wang R, Wang D. A study on LiFePO4/graphite cells with built-in Li4Ti5O12 reference electrodes. RSC Adv. 2018;8(33):18597.
Li Y, Han X, Feng X, Chu Z, Gao X, Li R, Du J, Lu L, Ouyang M. Errors in the reference electrode measurements in real lithium-ion batteries. J Power Sources. 2021;481:228933.
Chu Z, Feng X, Liaw B, Li Y, Lu L, Li J, Han X, Ouyang M. Testing lithium-ion battery with the internal reference electrode: an insight into the blocking effect. J Electrochem Soc. 2018;165(14):A3240–8.
Klett M, Gilbert JA, Trask SE, Polzin BJ, Jansen AN, Dees DW, Abraham DP. Electrode behavior RE-visited: monitoring potential windows, capacity loss, and impedance changes in Li1.03(Ni0.5Co0.2Mn0.3)097O2/silicon-graphite full cells. J Electrochem Soc. 2016;163(6):A875.
Ender M, Illig J, Ivers-Tiffée E. Three-electrode setups for lithium-ion batteries I. Fem-simulation of different reference electrode designs and their implications for half-cell impedance spectra. J Electrochem Soc. 2017;164(2):A71.
Costard J, Ender M, Weiss M, Ivers-Tiffée E. Three-electrode setups for lithium-ion batteries: II. Experimental study of different reference electrode designs and their implications for half-cell impedance spectra. J Electrochem Soc. 2017;164(2):A80.
Ender M, Weber A, Ivers-Tiffee E. Analysis of three-electrode setups for AC-impedance measurements on lithium-ion cells by FEM simulations. J Electrochem Soc. 2012;159(2):128.
Tanim TR, Dufek EJ, Dickerson CC, Wood SM. Electrochemical quantification of lithium plating: challenges and considerations. J Electrochem Soc. 2019;166(12):A2689.
Petzl M, Danzer MA. Nondestructive detection, characterization, and quantification of lithium plating in commercial lithium-ion batteries. J Power Sources. 2014;254:80.
Ringbeck F, Rahe C, Fuchs G, Sauer DU. Identification of lithium plating in lithium-ion batteries by electrical and optical methods. J Electrochem Soc. 2020;167(9):090536.
Petzl M, Kasper M, Danzer MA. Lithium plating in a commercial lithium-ion battery—a low-temperature aging study. J Power Sources. 2015;275:799.
Koleti UR, Dinh TQ, Marco J. A new on-line method for lithium plating detection in lithium-ion batteries. J Power Sources. 2020;451:227798.
Bond T, Zhou J, Cutler J. Electrode stack geometry changes during gas evolution in pouch-cell-type lithium ion batteries. J Electrochem Soc. 2017;164(1):A6158.
Bommier C, Chang W, Lu Y, Yeung J, Davies G, Mohr R, Williams M, Steingart D. In operando acoustic detection of lithium metal plating in commercial LiCoO2/graphite pouch cells. Cell Rep Phys Sci. 2020;1(4):100035.
Chang W, Mohr R, Kim A, Raj A, Davies G, Denner K, Park JH, Steingart D. Measuring effective stiffness of Li-ion batteries: via acoustic signal processing. J Mater Chem A. 2020;8(32):16624.
Hsieh YC, Leißing M, Nowak S, Hwang BJ, Winter M, Brunklaus G. Quantification of dead lithium via in situ nuclear magnetic resonance spectroscopy. Cell Rep Phys Sci. 2020;1(8):100139.
Sathiya M, Leriche JB, Salager E, Gourier D, Tarascon JM, Vezin H. Electron paramagnetic resonance imaging for real-time monitoring of Li-ion batteries. Nat Commun. 2015;6:6276.
Heenan TMM, Tan C, Hack J, Brett DJL, Shearing PR. Developments in X-ray tomography characterization for electrochemical devices. Mater Today. 2019;31:69.
Waldmann T, Iturrondobeitia A, Kasper M, Ghanbari N, Aguesse F, Bekaert E, Daniel L, Genies S, Gordon I, Löble M, De Vito E. Review—post-mortem analysis of aged lithium-ion batteries: disassembly methodology and physico-chemical analysis techniques. J Electrochem Soc. 2016;163(10):A2149.
Mühlbauer MJ, Dolotko O, Hofmann M, Ehrenberg H, Senyshyn A. Effect of fatigue/ageing on the lithium distribution in cylinder-type Li-ion batteries. J Power Sources. 2017;348:145.
Zinth V, Von Lüders C, Hofmann M, Hattendorff J, Buchberger I, Erhard S, Rebelo-Kornmeier J, Jossen A, Gilles R. Lithium plating in lithium-ion batteries at sub-ambient temperatures investigated by in situ neutron diffraction. J Power Sources. 2014;271:152.
von Lüders C, Zinth V, Erhard SV, Osswald PJ, Hofmann M, Gilles R, Jossen A. Lithium plating in lithium-ion batteries investigated by voltage relaxation and in situ neutron diffraction. J Power Sources. 2017;342:17.
Renfrew SE, McCloskey BD. Quantification of surface oxygen depletion and solid carbonate evolution on the first cycle of LiNi0.6Mn0.2Co0.2O2 electrodes. ACS Appl Energy Mater. 2019;2(5):3762.
McShane EJ, Colclasure AM, Brown DE, Konz ZM, Smith K, McCloskey BD. Quantification of inactive lithium and solid-electrolyte interphase species on graphite electrodes after fast charging. ACS Energy Lett. 2020;5(6):2045.
Acknowledgements
This study was financially supported by the National Key R&D Program of China (No.2016YFB0100300) and the National Natural Science Foundation of China (No. U1864213).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, XL., Liu, XH., Xie, WL. et al. Multiscale observation of Li plating for lithium-ion batteries. Rare Met. 40, 3038–3048 (2021). https://doi.org/10.1007/s12598-021-01730-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01730-3