Abstract
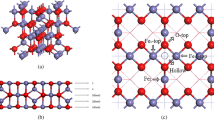
Fe-based solid catalysts in promoting Fenton reaction to generate ·OH radical has drawn much attention, and interestingly, FeOCl was reported to have superior activity compared with the traditional Fe2O3 catalysts. However, the mechanism of Fenton reaction on FeOCl and the origin of high activity remain unclear. Herein, by virtue of DFT + U calculations, the H2O2 decomposition and conversion mechanism on FeOCl(100) surface were systematically investigated. It is found that on clean FeOCl(100) surface, the exposed [Fe3+–Fe3+] sites can hardly break O–O bond of H2O2 into OH groups, but instead H2O2 tends to dehydrogenate by the surface lattice O, resulting in a series of side reactions and final conversion into O2, while the left H atoms gradually saturate the surface lattice O and reduce Fe3+ into Fe2+. On fully H-covered FeOCl(100), H2O2 can efficiently dissociate at [Fe2+–Fe2+] sites into two OH, but OH binds with Fe2+ so strongly that it cannot desorb as ·OH radical as easily as that on Fe3+. Interestingly, FeOCl(100) tends to be partially protonated in the real acid solution, which, along with H2O2 dehydrogenation, results in the formation of active unit [Fe2+–Fe3+]. On [Fe2+–Fe3+] unit, H2O2 can easily break its O–O bond and OH at Fe3+ can desorb as ·OH radical, while the other OH at Fe2+ couples with the surface H into H2O and finish the catalytic cycle. By comparison, Fe2O3(012) cannot provide enough [Fe2+–Fe3+] active units due to the relative difficulty in H2O2 dehydrogenation, which accounts for its inferior catalytic efficiency for Fenton reaction.








Similar content being viewed by others
References
Martínez-Huitle CA, Brillas E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B Environ. 2009;87(3):105.
Brillas E, Sirés I, Oturan MA. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev. 2009;109(12):6570.
Chen R, Pignatello JJ. Role of quinone intermediates as electron shuttles in Fenton and photoassisted Fenton oxidations of aromatic compounds. Environ Sci Technol. 1997;31(8):2399.
Chamarro E, Marco A, Esplugas S. Use of Fenton reagent to improve organic chemical biodegradability. Water Res. 2001;35(4):1047.
Wang FC, Zhao JM, Wang WK, Tong ZZ. Adsorption of Au(III) by amino-modified monodispersed PGMA microspheres and deposition of gold nanoparticles. Rare Met. 2018;37(3):196.
Andreozzi R, Caprio V, Insola A, Marotta R, Sanchirico R. Advanced oxidation processes (AOP) for water purification and recovery. Catal Today. 1999;53(1):51.
Pignatello JJ, Oliveros E, MacKay A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol. 2006;36(1):1.
De Morais JL, Zamora PP. Use of advanced oxidation processes to improve the biodegradability of mature landfill leachates. J Hazard Mater. 2005;123(1):181.
Ayoub K, van Hullebusch ED, Cassir M, Bermond A. Application of advanced oxidation processes for TNT removal: a review. J Hazard Mater. 2010;178(1):10.
Feng L, van Hullebusch ED, Rodrigo MA, Esposito G, Oturan MA. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem Eng J. 2013;228(1):944.
Enami S, Sakamoto Y, Colussi AJ. Fenton chemistry at aqueous interfaces. PNAS. 2014;111(2):623.
Neyens E, Baeyens J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater. 2003;98(1):33.
Ventura A, Jacquet G, Bermond A, Camel V. Electrochemical generation of the Fenton’s reagent: application to atrazine degradation. Water Res. 2002;36(14):3517.
Zepp RG, Faust BC, Hoigne J. Hydroxyl radical formation in aqueous reactions (pH 3–8) of iron (II) with hydrogen peroxide: the photo-Fenton reaction. Environ Sci Technol. 1992;26(2):313.
Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82(6):969.
Garrido-Ramírez EG, Theng BKG, Mora ML. Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions—a review. Appl Clay Sci. 2010;47(3):182.
Wei H, Cai Q, Rahn RO. Inhibition of UV light-and Fenton reaction-induced oxidative DNA damage by the soybcan isoflavone genistein. Carcinogenesis. 1996;17(1):73.
Zhou T, Li YZ, Ji J. Oxidation of 4-chlorophenol in a heterogeneous zero valent iron/H2O2 Fenton-like system: kinetic, pathway and effect factors. Sep Purif Technol. 2008;62(3):551.
Ruppert G, Bauer R, Heisler G. The photo-Fenton reaction-an effective photochemical wastewater treatment process. J Photochem Photobiol, A. 1993;73(1):75.
Cohen G. Enzymatic nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann NY Acad Sci. 1994;738(1):8.
Huang YF, Huang YH. Identification of produced powerful radicals involved in the mineralization of bisphenol A using a novel UV–Na2S2O8/H2O2–Fe(II, III) two-stage oxidation process. J Hazard Mater. 2009;162(2):1211.
Yao YY, Wang L, Zhu S, Huang ZF, Mao YJ, Lu WY, Chen WX. Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity. Chem Eng Sci. 2013;101(14):424.
Zhou M, Yu Q, Lei L, Barton G. Electro-Fenton method for the removal of methyl red in an efficient electrochemical system. Sep Purif Technol. 2007;57(2):380.
Ai ZH, Cheng Y, Zhang LZ, Qiu JR. Efficient removal of Cr(VI) from aqueous solution with Fe@Fe2O3 core-shell nanowires. Environ Sci Technol. 2008;42(18):6955.
Huang R, Fang Z, Yan X, Cheng W. Heterogeneous sono-Fenton catalytic degradation of bisphenol A by Fe3O4 magnetic nanoparticles under neutral condition. Chem Eng J. 2012;197(14):242.
Zhao YP, Hu JY, Chen HB. Elimination of estrogen and its estrogenicity by heterogeneous photo-Fenton catalyst β-FeOOH/resin. J Photochem Photobiol, A. 2010;212(2):94.
Shahwan T, Abu Sirriah S, Nairat M, Boyacı E, Eroğlu AE, Scott TB, Hallam KR. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem Eng J. 2011;172(1):258.
Rajeshwar K, Osugi ME, Chanmanee W, Chenthamarakshan CR, Zanoni MVB, Kajitvichyanukul P, Krishnan-Ayer R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J Photochem Photobiol, C. 2008;9(4):171.
Yang SJ, He HP, Wu DQ, Chen D, Liang XL, Qin ZH, Fan MD, Zhu JX, Yuan P. Decolorization of methylene blue by heterogeneous Fenton reaction using Fe3−xTixO4 (0≤x≤0.78) at neutral pH values. Appl Catal B Environ. 2009;89(3):527.
Zhang GK, Gao YY, Zhang YL, Guo YD. Fe2O3-pillared rectorite as an efficient and stable Fenton-like heterogeneous catalyst for photodegradation of organic contaminants. Environ Sci Technol. 2010;44(16):6384.
Pouran SR, Raman AAA, Daud WMAW. Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J Clean Prod. 2014;64(2):24.
Yang XJ, Xu XM, Xu J, Han YF. Iron oxychloride (FeOCl): an efficient Fenton-like catalyst for producing hydroxyl radicals in degradation of organic contaminants. J Am Chem Soc. 2013;135(43):16058.
Kuo WG. Decolorizing dye wastewater with Fenton’s reagent. Water Res. 1992;26(7):881.
Kremer ML. Mechanism of the Fenton reaction. Evidence for a new intermediate. Phys Chem Chem Phys. 1999;1(15):3595.
Aleksić M, Kušić H, Koprivanac N, Leszczynska D, Božić AL. Heterogeneous Fenton type processes for the degradation of organic dye pollutant in water—the application of zeolite assisted AOPs. Desalination. 2010;257(1):22.
Costa RCC, Lelis MFF, Oliveira LCA, Fabris JD, Ardisson JD, Rios RRVA, Silva CN, Lago RM. Novel active heterogeneous Fenton system based on Fe3−xMxO4 (Fe Co, Mn, Ni): the role of M2+ species on the reactivity towards H2O2 reactions. J Hazard Mater. 2006;129(1):171.
Costa RC, Moura FC, Ardisson JD, Fabris JD, Lago RM. Highly active heterogeneous Fenton-like systems based on Fe0/Fe3O4 composites prepared by controlled reduction of iron oxides. Appl Catal B Environ. 2008;83(1):131.
Kresse G, Hafner J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J Phys: Condens Matter. 1994;6(40):8245.
Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci. 1996;6(1):15.
Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B. 1999;59(3):1758.
Alavi A, Hu PJ, Deutsch T, Silvestrelli PL, Hutter J. CO oxidation on Pt(111): an ab initio density functional theory study. Phys Rev Lett. 1998;80(16):3650.
Liu ZP, Hu P. General rules for predicting where a catalytic reaction should occur on metal surfaces: a density functional theory study of C–H and C–O bond breaking/making on flat, stepped, and kinked metal surfaces. J Am Chem Soc. 2003;125(7):1958.
Wang HF, Kavanagh R, Guo YL, Guo Y, Lu GZ, Hu P. Structural origin: water deactivates metal oxides to CO oxidation and promotes low-temperature CO oxidation with metals. Angew Chem Int Ed. 2012;51(27):6657.
Wang HF, Wang D, Liu XH, Guo YL, Lu GZ, Hu P. Unexpected C–C bond cleavage mechanism in ethylene combustion at low temperature: origin and implications. ACS Catal. 2016;6(8):5393.
Wang D, Wang HF, Hu P. Identifying the distinct features of geometric structure for hole trapping to generate radicals on rutile TiO2(110) in photooxidation using density functional theory calculations with hybrid functional. Phys Chem Chem Phys. 2015;17(3):1549.
Zhang JW, Peng C, Wang HF, Hu P. Identifying the role of photogenerated holes in photocatalytic methanol dissociation on Rutile TiO2(110). ACS Catal. 2017;7(4):2374.
Cococcioni M, de Gironcoli S. Linear response approach to the calculation of the effective interaction parameters in the LDA+U method. Phys Rev B. 2005;71(3):035105.
Rollmann G, Rohrbach A, Entel P, Hafner J. First-principles calculation of the structure and magnetic phases of hematite. Phys Rev B. 2004;69(16):165107.
Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory. J Comput Chem. 2011;32(7):1456.
Wasserman E, Rustad JR, Felmy AR, Hay BP, Halley JW. Ewald methods for polarizable surfaces with application to hydroxylation and hydrogen bonding on the (012) and (001) surfaces of α-Fe2O3. Surf Sci. 1997;385(2–3):217.
Guo Y, Clark SJ, Robertson J. Electronic and magnetic properties of Ti2O3, Cr2O3, and Fe2O3 calculated by the screened exchange hybrid density functional. J Phys: Condens Matter. 2012;24(32):325504.
Henkelman G, Arnaldsson A, Jónsson H. A fast and robust algorithm for Bader decomposition of charge density. Comput Mater Sci. 2006;36(3):354.
Mathew K, Sundararaman R, Letchworth-Weaver K, Arias TA, Hennig RG. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J Chem Phys. 2014;140(8):084106.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21622305 and 21333003), the Young Elite Scientist Sponsorship Program by China Association for Science and Technology (No. YESS20150131), “Shu Guang” Project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (No. 17SG30) and the Fundamental Research Funds for the Central Universities (No. WJ1616007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, XX., Wang, HF. & Hu, PJ. First principles study of Fenton reaction catalyzed by FeOCl: reaction mechanism and location of active site. Rare Met. 38, 783–792 (2019). https://doi.org/10.1007/s12598-018-1140-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-018-1140-9