Abstract
Al2O3-coated spinel LiMn2O4 cathode materials, presintered LiMn2O4 (P-LMO), and calcined LiMn2O4 (C-LMO) were synthesized by chemical deposition and thermal treating method using presintered and calcined LiMn2O4 as precursors. The crystal structure, morphology, the thickness of the coating layer, and particle size of prepared samples were investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), and Malvern instruments. The average particle size of P-LMO with like-spheres (0.3 μm) is much smaller than that of C-LMO (0.5 μm). The Al2O3 layer of P-LMO can effectively reduce the charge transfer resistance and inhibit the Mn dissolution. The electrochemical performance of P-LMO is better than that of C-LMO. It is found that the LiMn2O4 cathode materials have excellent electrochemical cyclability by coated 2 mol% Al2O3 at the surface of presintered material. The initial discharge capacity of the material with 2 mol% Al2O3-coated is 114.0 mAh·g−1 at 0.1C rate and 55 °C, and the capacity retention is 87.3 % at 0.5C rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Spinel LiMn2O4, owing to its easy preparation, economic, and environmental advantages, is considered to be a promising material for the positive electrode of secondary lithium batteries [1–3]. The compound has significant advantages in terms of toxicity and cost over LiCoO2. Moreover, the natural abundance of manganese and the familiarity of battery manufacturer with the chemistry of manganese oxides, used in primary alkaline cells [4], made the choice of LiMn2O4 even more attractive.
The capacity of spinel LiMn2O4, however, fades rapidly during charge/discharge cycling, which seriously hinders the commercial application of spinel LiMn2O4 materials. Several possible reasons were proposed to contribute to this fading, such as Jahn–Teller distortion [5], dissolution of spinel into the electrolyte, and decomposition of the electrolyte [6] during cycling and oxygen loss from the spinel lattice of cathode material [7]. Extensive studies were directed toward various strategies to overcome the capacity fading problem of LiMn2O4. Elemental substitution of Mn with transition metals, such as Zr, Co, Al, Cr, Ni, Mg, etc. [8–12], was proven to be an effective method. However, the improved capacity retention is usually realized at the expense of the decrease of specific capacity.
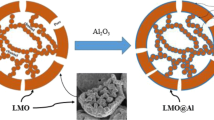
Morphology, surface chemistry, surface species of the cathodes and the interface between cathodes and electrolyte are of great importance to the electrochemical performance of lithium-ion batteries. Oxides such as MgO, TiO2, CeO2, ZnO, ZrO2, and Al2O3 [13–17], were studied to suppress capacity fading, indicating that the effectiveness of surface modification on spinel LiMn2O4. Al2O3 was improved to be one of the surface coating materials for lithium-ion batteries because of its various advantages such as low cost, excellent chemical stability, and high electronic conductivity possessed by amphoteric oxides [18]. Currently, most of the studies focus on the coating methods of Al2O3. Tu et al. [19] reported that the Al2O3 layer was coated through a melting impregnation method, and Kim et al. [20] found Al2O3 could coat on the spinel LiMn2O4 by electrostatic attraction forces. On the basis of preliminary studies, Al(OH)3 layer was prepared on the surface of presintered LiMn2O4 by a chemical deposition method, and then it was thermally treated to form Al2O3 layer. The Al2O3 layer can inhibit the growth of LiMn2O4 during the progress of calcination and reduce the particle size. Moreover, the layer can effectively isolate the LiMn2O4 and electrolyte, reduce the contact area and diffusion of Mn in the electrolyte, and further improve the electrochemical performance of spinel LiMn2O4 cathode materials. However, there are few reports on the preparation of Al2O3-coated LiMn2O4 using the presintered LiMn2O4 as precursor. In this work, presintered LiMn2O4(P-LMO) and calcined LiMn2O4(C-LMO) coated with different amounts of Al2O3 were prepared, and the effects of Al2O3 coating on the electrochemical cycling performances of LiMn2O4 were systematically studied.
2 Experimental
LiMn2O4 was synthesized by solid-state method. The stoichiometric amounts of Li2CO3 and electrochemically prepared manganese dioxide (EMD) were firstly mixed in ball grinding mill for 16 h. After the mixture was dried at 80 °C, it was sintered at 500 °C for 6 h to obtain presintered LiMn2O4, and then calcined at 750 °C for 6 h to gain calcined LiMn2O4.
The Al2O3 coating level was controlled at 2 mol% and 5 mol%, and the preparation process of coating solution was conducted as follows. Certain amounts of presintered LiMn2O4 and calcined LiMn2O4 were homogeneously dispersed in the absolute ethyl alcohol, respectively. The solutions of aluminum nitrate and aqueous ammonia were slowly added into the dispersion successively drop by drop with electromagnetic stirring in a water bath at 80 °C for several hours, and then Al(OH)3-coated presintered LiMn2O4 and calcined LiMn2O4 were obtained, respectively, after the solution was evaporated. Finally, the Al(OH)3-coated presintered LiMn2O4 and calcined LiMn2O4 powers were annealed in air at 750 and 350 °C for 10 h, respectively, to obtain Al2O3-coated LiMn2O4. According to different Al2O3 coating amounts, pristine LiMn2O4, Al2O3 cal-coated LiMn2O4, and Al2O3 pre-coated LiMn2O4 were labeled as LMO, C-LMO-2, C-LMO-5, P-LMO-2, and P-LMO-5.
The working electrode was prepared by pressing a mixing of the cathode active material, conductive material (acetylene black), and binder (PVDF) in a weight ratio of 80/10/10. The slurry was then pasted on the aluminum foil current collector and dried at 120 °C under vacuum for overnight. The Li metal was used as the counter and reference electrodes. The electrolyte consisted of 1 mol·L−1 solution of LiPF6 dissolved in an ethylene/diethyl carbonate (EC/DEC, 1:1 ratio by volume). The cells were assembled in argon-filled dry box in which both the moisture and the oxygen levels were less than 1 × 10−6.
Power X-ray diffraction (XRD, SIEMENSD-500) using Cu Kα radiation was used to examine the crystalline phase and the evolution lattice parameters of the prepared material. The surface morphology and microstructure of powers were characterized by scanning electron microscopy (SEM, Sirion 200) and high-resolution transmission electron microscopy (HRTEM, JEM-2100F). The distribution of solid particles was determined using a ZetaSizer 3,000 dynamic light scattering instrument (Malvern Inc.). Before electrochemical tests, the batteries were aged for 24 h to ensure good soakage. The cells fabricated were cycled galvanostatically at 0.1C and 0.5C in the potential range of 3.3–4.5V. Alternating current (AC) impedance was carried out by applying frequency of 1 × 105–1 × 10−2 Hz with ac-amplitude of 5 mV. Cyclic voltammogram (CV) measurements were performed on electrochemical workstation with three electrode systems (CHI660C, Shanghai), and the CV curves were recorded at scan rate of 0.1 mV·s−1 between 3.3 and 4.5 V (vs. Li/Li+).
In order to investigate the effect of Al2O3 coating layer on decreasing the dissolution of cathode material, the Mn dissolution experiment was also carried out in this study according to the method given in Ref. [21]. About 10 mg of LMO, P-LMO, and C-LMO were, respectively, immersed in 10 ml of LiPF6-EC/DEC (1 mol·L−1, 1:1 volume ratio) electrolyte for 3 days. Then, after separating the cathode material power via filtering through filter paper, the dissolved Mn2+ in the electrolyte was extracted into water phase using 10 ml HCl (0.1 mol·L−1) acid, and the Mn contents were quantitatively determined by atomic absorption spectroscopy (AAS).
3 Results and discussion
3.1 Physical characterizations
Figure 1 displays the XRD patterns of the LMO, C-LMO, and P-LMO samples with different Al2O3 coating amounts. Figure 1 indicates that all of the diffraction peaks correspond to a well-defined spinel structure with space group Fd3m, being in good agreement with JCPDS card 88-1,749. The absence of any other peaks in the patterns indicates that amorphous Al2O3 coating on the surface of the base LiMn2O4 does not penetrate the spinel matrix.
Compared with the (440) diffraction peak of pristine LiMn2O4, as shown in the inset of Fig. 1, all the peaks of the Al2O3-coated LiMn2O4 exhibit a minute leftward shift, demonstrating that the crystal lattice of LiMn2O4 is enlarged after coating a layer of Al2O3. As can be seen from the inset, the lattice constants for both C-LMO and P-LMO increase with the increase of coating amount of Al2O3. And for the same amount of Al2O3 surface-modified spinel LiMn2O4, the (440) diffraction peaks of C-LMO have a further leftward than those of P-LMO, which indicates that P-LMO has smaller lattice parameter. So we suggest that the Al2O3 layer coated at presintering stage can successfully prohibit grain from growing up. Secondary particles are composed of small grains which further confirm the inhibition of Al2O3 layer to spinel LiMn2O4. The particle size measurement of P-LMO-2 and C-LMO-2 was conducted with a laser particle size analyzer.
The particle diameter distributions of P-LMO-2 and C-LMO-2 are presented in Fig. 2. It can be seen from Fig. 2 that the average particle sizes of P-LMO and C-LMO are about 0.30 and 0.50 μm, respectively, and the grain size distribution of P-LMO is more concentrated. It may be attributed to the fact that the grain is growing by intergranular annexed to each other and through the migrating of the grain boundary. Because there is a layer of alumina hydroxide coated at the surface of presintered spinel LiMn2O4 samples before calcinations, the atoms only can grow up in the cladding layer and not by eating grains outside of the cladding layer during the process of calcinations. This is different from the growing way of LiMn2O4-coated Al2O3 after calcinations [22], so the particle diameter of P-LMO is much smaller. This indicates that pre-coated Al2O3 can reduce the size of particles and also narrow the distribution range of particle size.
The SEM images of the surface morphology of LMO, C-LMO, and P-LMO samples are presented in Fig. 3. The SEM images reveal that the particles of pristine LiMn2O4 are homogeneously distributed with average particle diameter of 200–300 nm. The border and corner of particles are clearly observed. After surface coating with 2 mol% Al2O3 (Fig. 3b, c) and 5 mol% Al2O3 (Fig. 3d, e), the following conclusions can be obtained. Firstly, C-LMO powders exhibit well-developed polyhedral morphology, and the border and corner of the particles are smoother than those of spinel LiMn2O4. In contrast, the particle morphology of P-LMO shows spherical shape and the clear border and corner of particles disappear. Secondly, the particle size of P-LMO is smaller than that of C-LMO, which is consistent with the results of particle diameter distribution.
HRTEM was applied to characterize what was changed on the particle surface after surface modification. Figure 4 shows the images of LMO, P-LMO-2, and C-LMO-2 particles. From Fig. 4a, it is evident that LiMn2O4 grains are submicron particles with good dispersivity. The average size of these particles ranges from 200 to 300 nm. For both coated samples (Fig. 4b–e), core LiMn2O4 (dark opaque region) can be clearly seen covered by loose thin translucent film. The thickness of the Al2O3 compact coating layer is around 10 nm. No transition layer can be observed, and the interface between the core and coating layer of C-LMO (Fig. 4d) is obvious. However, the interface between the core and coating layer of P-LMO (Fig. 4e) is unobvious. This may be due to that the Al(OH)3 coating layer of the core presintered LiMn2O4 gradually diffuses into the LiMn2O4 crystal and reacts with it, resulting in the formation of solid solution layer during the sintering process. However, the relative intensity of diffraction peaks has no significant change between LMO and P-LMO, indicating that only a small amount Al3+ embed into the crystal on the surface of particles, and then the Li–Al–Mn–O solid solution which is similar to a body-doped LiAlyMn2-yO4 forms on the surface [23].
3.2 Electrochemical performance
Figure 5 shows the initial specific discharge capacities of LMO, C-LMO, and P-LMO by applying a current of 0.1C at the potential range of 3.3–4.5 V (vs. Li/Li+) at room temperature.
It can be seen from Fig. 5 that the discharge curves of all samples have two voltage plateaus at approximately 3.9 and 4.1 V. The first plateau at 3.9 V is associated with the single-phase reversible reaction of LiMn2O4 → Li0.5Mn2O4 + 0.5Li+, while the second one at 4.1 V is attributed to the two-phase reaction of Li0.5Mn2O4 → Mn2O4 + 0.5Li+ [21]. The initial discharge capacities of LMO, C-LMO, and P-LMO at room temperature are shown in Table 1. From Table 1, it can be seen that the initial specific discharge capacities of Al2O3-coated LiMn2O4 are nearly synonymous with that of LMO. This indicates that the layer of Al2O3 has no effect on the initial specific discharge capacity of spinel LiMn2O4, and a small amount of Al3+ embedded into the crystal on the surface of P-LMO do not cause the loss of the initial capacity. The two potential plateaus for all P-LMO samples are maintained after 50th cycling, but 5 mol% C-LMO only shows one voltage plateau at the cutoff voltage as shown in Fig. 6. The discharge plateaus of C-LMO descend, and the two platforms integrate gradually during the recycling process. This may be due to that a small amount of Al3+ embed into the crystal lattice of LiMn2O4 and the stronger Al–O bond (bond energy of 512 kJ·mol−1) weakens Li–O bond (bond energy of 340.6 kJ·mol−1), making the lithium ions more easily spread the 8a-16c-8a channel, so the discharge performance of P-LMO samples is better [24]. However, the layer coated at calcined LiMn2O4 does not have the above function, and the discharge capacity fades rapidly. This is in part due to the disproportionation reaction of Mn3+ in the electrolyte: Mn3+ (insoluble) → Mn4+ (insoluble) + MnO (soluble). The dissolved Mn2+ is reduced and deposited on the cathode surface in the form of manganese, which leads to the increase of resistance. And the other part is that the excessive Al2O3 seriously restraints the insertion and extraction of lithium ions with the increase of Al2O3 coating amount. Both of them can cause polarization of the charge–discharge curves [25].
Figure 7 shows the cycling performances of LMO, C-LMO, and P-LMO in the voltage range of 3.3–4.5 V by applying a current of 0.5C at 55 °C. As for LMO, the specific discharge capacity fades from 96.7 to 64.4 mAh·g−1 after 50 cycles, with capacity loss of 33.4 %. In the case of P-LMO, the specific discharge capacities decrease from 100.9 to 88.1 mAh·g−1 and 99.5 to 86.8 mAh·g−1 with capacity loss of 12.7 % and 12.8 % for the coating contents of 2 mol% and 5 mol%, respectively. And the capacity loss of corresponding C-LMO is 23.6 % and 27.5 %. Obviously, the cycling performance of P-LMO is superior to those of LMO and C-LMO material. It is attributed to the following reasons: (1) the layer coated at presintering period narrows the particle size range and facilitates the diffusion of lithium ions; 2) the small lattice constants help stabilize the structure of spinel crystal, and thus inhibiting the Jahn–Teller distortion accompanied by the change of electrode material from cubic to tetragonal; 3) the Al2O3 layer helps improve the valence of Mn ions on the surface of (001) and can effectively reduce the dissolution of Mn3+ on the surface of the electrode as well as the chemical reaction between Mn3+ and electrolyte [26]. In addition, researches show that P-LMO-2 has a better cycle performance than P-LMO-5, which is due to that the excess Al2O3 coated at P-LMO-5 will hinder the transportation of lithium ions, leading to the evident decay of the specific capacity.
In order to further understand the difference in their cyclic performance, electrochemical impedance spectroscopy (EIS) of pristine LiMn2O4- and Al2O3-coated LiMn2O4 at the 1st cycle and 50th cycle were measured as shown in Fig. 8. Figure 8a shows the equivalent circuit of the cell to simulate film resistance (Rf), charge transfer resistance (Rct), Warburg resistance (Zw), and capacitor element (CPE). The Rf and Rct data are listed in Table 2. From the results of the 1st cycle, the Rf values of P-LMO and C-LMO are larger than that of LMO due to the combination of the solid electrolyte interface (SEI) and Al2O3 coating layer on the surface of LiMn2O4. The charge transfer resistance (Rct) of P-LMO has the slowest growth rate; and when the Al2O3 coating amount is 5 mol%, the Rct grows rapidly for both P-LMO and C-LMO. Similar situations appear in the results of the 50th cycle. This is mainly due to the dissolution of Mn ions, declining the instability of the spinel structure and further resulting in a rapid increase in impedance. However, the Al2O3 coating layer at the surface of LiMn2O4 can separate electrolytes and active materials to decrease the decomposition of electrolyte. Particularly, the LiAlyMn2-yO4 formed on the LiMn2O4 surface in the P-LMO power strengths the spinel structure and further decreases the growth rate of resistance. In addition, the excess Al2O3 coated at P-LMO and C-LMO will hinder the transportation of lithium ions, leading to the rise of resistance.
In Fig. 9a and b, the CV profiles of Li/P-LMO-2 were compared with the Li/C-LMO-2 cells in the 1st and 50th cycles at room temperature. Figure 9 indicates that the intervals between the oxidation and the corresponding reduction potential of P-LMO are smaller than those of the C-LMO, indicating that the polarization of the cathode material decreases. CVs for the Li/C-LMO-2 show a distinct shift and broadening of the oxidation and reduction peaks during cycling, indicating a change in the surface structure and composition of the spinel electrode. These changes are likely associated with the rapid capacity losses. By contrast, the CVs of Li/P-LMO-2 show significantly less peak shift and broadening, implying a quick electrode reaction after Al2O3 pre-coated. The results are consistent with our conclusion that the Al2O3 pre-coating plays an important role in protecting the electrode surface from chemical attack by acidic electrolyte species, such as HF; thereby, the structural and chemical character of the electrode surface are maintained. In addition, the CVs of C-LMO products appear a redox peak among the voltage of 3.1–3.2 V, while it does not occur in the pre-coated ones. This is mainly attributed to the aluminum hydroxide layer coated at the surface of presintered LiMn2O4. The layer can effectively avoid the generation of oxygen defects at a high temperature during the calcination process.
AAS results for the Mn dissolution of LMO, C-LMO, and P-LMO cathode materials are given in Fig. 10. The dissolved Mn contents of P-LMO and C-LMO are much lower than that of LMO powers, and that of the P-LMO is the lowest. As a result, it is demonstrated that both cal-coating and pre-coating of Al2O3 could effectively reduce the dissolution of Mn from the electrode, but the solution-based Al2O3 pre-coating is more effective in the reduction of the Mn dissolution into the electrolyte.
3.3 Mechanism exploration
Some researchers believed that the fast capacity loss during cycling for spinel LiMn2O4 was mainly ascribed to the dissolution of manganese from the electrode. HF generated during cycling when using LiPF6-based electrolyte was responsible for the dissolution of the manganese. This chemical reaction was proposed by Myung et al. [27]:
HF continuously attacks the active cathode material, and cathode material decomposes as the cycle goes by [28], causing capacity fading
Therefore, it is believed that the uniform layer coated at spinel LiMn2O4 can effectively prevent cathode materials from direct contacting with the electrolyte, reduce the formation of HF, and greatly decrease the capacity loss. The electrochemical data obtained in this study demonstrate that the cycling performance of LiMn2O4 electrode is significantly improved by pre-coating Al2O3 layer. The layer coated at the surface of presintered LiMn2O4 can inhibit the growth of LiMn2O4 during the further process of calcination and reduce the particle sizes. The small size is contributed to the diffusion of lithium ions. Owing to the decrease of diffusion distance, the utilization of the active material is improved. And P-LMO can also avoid the shortcoming of traditional C-LMO obtained by coating calcined LiMn2O4, in which the particles near the center of active materials are difficult to use. Moreover, the Al2O3 layer can effectively decrease the interface area between the cathode and the electrolyte as well as the HF content at the cathode surface.
Pre-coating the spinel LiMn2O4 with Al2O3 layer via chemical deposition method results in the improvement of the electrochemical properties.
4 Conclusion
Spinel LiMn2O4 was synthesized by the two-process solid-state method, and the Al2O3 coating layer was coated on the surface of presintering LiMn2O4 and calcined LiMn2O4 by chemical deposition method. Al2O3 layer forms uniformly on the surface of spinel LiMn2O4. Compared with C-LMO, P-LMO materials have smaller particle size and show much batter capacity retention at 55 °C, especially the 2 mol% Al2O3 pre-coated LiMn2O4. The capacity retention of this material is 87.3 % after 50 cycles. The surface modification with Al2O3 coated at presintering period in this work acts at least two roles: (1) preventing the particles further excessively growing and facilitating the diffusion of lithium ions through it, and (2) separating electrolytes and active materials and thus preventing the dissolution of the Mn ions to ensure the structure stability. Furthermore, this pre-coated way combined with traditional solid-state method makes the industrialization of cathode material LiMn2O4 for lithium-ion batteries more prosperous.
References
Xu B, Qian DN, Wang ZY, Meng YS. Recent progress in cathode materials research for advanced lithium ion batteries. Mater Sci Eng. 2012;73(5–6):51.
Wu HM, Tu JP, Chen XT, Li Y, Zhao XB, Cao GS. Electrochemical study on LiMn2O4 as cathode material for lithium ion batteries. J Electroanal Chem. 2006;586(2):180.
He XM, Li JJ, Cai Y, Jiang CY, Wan CR. Preparation of spherical spinel LiMn2O4 cathode material for Li-ion batteries. Mater Chem Phys. 2006;95(1):105.
Cabana J, Valdes-Solis T, Palacin MR, Oro-Sole J, Fuertes A, Marban G, Fuertes AB. Enhanced high rate performance of LiMn2O4 spinel nanoparticles synthesized by a hard-template route. J Power Sources. 2007;166(2):492.
Li XF, Xu YL, Wang CL. Suppression of Jahn-Teller distortion of spinel LiMn2O4 cathode. J Alloy Compd. 2009;479(1–2):310.
Xiao LF, Zhao YQ, Yang YY, Cao YL, Ai XP, Yang HX. Enhanced electrochemical stability of Al-doped LiMn2O4 synthesized by a polymer-pyrolysis method. Electrochim Acta. 2008;54(2):545.
Xia YG, Wang HY, Zhang Q, Nakamura H, Noguchi H, Yoshio M. Oxygen deficiency, a key factor in controlling the cycle performance of Mn-spinel cathode for lithium-ion batteries. J Power Sources. 2007;166(2):485.
Oh SH, Chung KY, Jeon SH, Kim CS, Cho WB. Structural and electrochemical investigations on the LiNi0.5-xMn1.5-yMx+yO4 (M = Cr, Al, Zr) compound for 5 V cathode material. J Alloy Compd. 2009;469(1–2):244.
Hwang BJ, Tsai YW, Santhanam R, Wu YW, Hu SG, Lee JF, Liu DG. Evolution of local electronic and atomic structure of Co-doped LiMn2O4 cathode material for lithium rechargeable batteries. J Power Sources. 2006;123(2):206.
Hwang BJ, Santhanam R, Liu DG, Tsai YW. Effect of Al-substitution on the stability of spinel LiMn2O4 synthesized by citric acid sol-gel method. J Power Sources. 2001;102(1–2):326.
Wei YJ, Nam KW, Kim KB, Chen G. Spectroscopic studies of the structural properties of Ni substituted spinel LiMn2O4. Solid State Ion. 2006;177(1–2):29.
Lee KS, Bang HJ, Myung ST, Prakash J, Amine K, Sun YK. Synthesis and electrochemical properties of spherical spinel Li1.05M0.05Mn1.9O4 (M = Mg and Al) as a cathode material for lithium-ion batteries by co-precipitation method. J Power Sources. 2007;174(2):726.
Walz KA, Johnson CS, Genthe J, Stoiber LC, Zeltner WA, Anderson MA, Thackeray MM. Elevated temperature cycling stability and electrochemical impendence of LiMn2O4 cathodes with nanoporous ZrO2 and TiO2 coating. J Power Sources. 2010;195(15):4943.
Gnanaraj JS, Pol VG, Gedanken A, Aurbach D. Improving the high-temperature performance of LiMn2O4 spinel electrodes by coating the active mass with MgO via a sonochemical method. Electrochem Commun. 2003;5(11):940.
Eftekhari A. Aluminum oxide as a multi-function agent for improving battery performance of LiMn2O4 cathode. Solid State Ion. 2004;167(3–4):237.
Tu J, Zhao XB, Xie J, Cao GS, Zhuang DG, Zhu TJ, Tu JP. Enhanced low voltage cycling stability of LiMn2O4 cathode by ZnO coating for lithium ion batteries. J Alloy Compd. 2007;432(1–2):313.
Ha HW, Yun NJ, Kim K. Improvement of electrochemical stability of LiMn2O4 by CeO2 coating for lithium-ion batteries. Electrochim Acta. 2007;52(9):3236.
Cui YL, Wu C, Wei T, Shi YL, Zhuang QC, Sun Z. Electrochemical performance of spinel LiMn2O4 modified. Hemistry. 2011;74(8):742.
Tu J, Zhao XB, Cao GS, Zhuang DG, Zhu TJ, Tu JP. Enhanced cycling stability of LiMn2O4 by surface modification with melting impregnation method. Electrochim Acta. 2006;51(28):6456.
Kim WK, Han DW, Ryu WH, Lim SJ, Kwon HS. Al2O3 coating on LiMn2O4 by electrostatic attraction forces and its effects on the high temperature cyclic performance. Electrochim Acta. 2012;71(5):17.
Halil S, Huseyin G, Saban P, Ahmet U. The effect of LBO coating method on electrochemical performance of LiMn2O4 cathode material. Solid State Ion. 2008;178(35–36):1837.
Zheng ZQ. Fundamentals of Materials Science. Changsha: Central South University; 2005. 365.
Wang ZX, Xing ZJ, Li XH, Guo HJ, Peng WJ. Study on Al2O3-modified LiMn2O4 prepared by heterogeneous nucleation. Acta Phys Chim Sin. 2004;20(8):790.
Xiong LL, Xu YL, Zhang C, Tao T. Doping-coating surface modification of spinel LiMn2O4 cathode material with Al3+ for lithium-ion batteries. Acta Phys Chim Sin. 2012;28(5):1177.
Duan F, Du ZZ, Yang P, Chen P, Zhong SS, Zhang DW. Study of coated as a cathode material for lithium ion batteries. J Hefei Univ Technol (Nat Sci). 2013;36(3):346.
Li XC, Zhang XY, Lu YJ, Li K. Interface reaction mechanism of welding Al2O3 ceramic with AgCuInTi brazing filler. Chin J Rare Met. 2013;37(1):71.
Myung ST, Izumi K, Komaba S, Sun YK, Yashiro H, Kumagai N. Role of alumina coating on Li-Ni-Co-Mn-O particles as positive electrode material for lithium-ion batteries. Chem Mater. 2005;17(14):3695.
Qing CB, Bai Y, Yang JM, Zhang WF. Enhanced cycling stability of LiMn2O4 cathode by amorphous FePO4 coating. Electrochim Acta. 2011;56(19):6612.
Acknowledgments
This study was financially supported by the Science and Technology Project of Hunan Province (No. 2010FJ4061) and the Technology Project of Changsha (No. K1201039-11).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Zhou, HM., Zhu, YH., Li, J. et al. Electrochemical performance of Al2O3 pre-coated spinel LiMn2O4. Rare Met. 38, 128–135 (2019). https://doi.org/10.1007/s12598-014-0418-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-014-0418-9