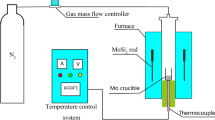
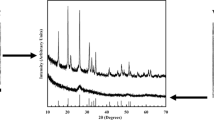
Abstract
Vanadium nitride was synthesized by one-step method using V2O5 and carbon black as raw materials in nitrogen atmosphere. The phases of different reaction products prepared in different reaction temperatures were analyzed by X-ray diffraction (XRD), and the dynamic behavior of the process of synthesizing vanadium nitride (VN) by one-step method was studied with non-isothermal thermogravimetry. The mechanism function and kinetic parameters of reaction process were calculated by thermal gravimetric analyses (TGA), and the reaction rate equation was established. The XRD results show that for the samples tested with minimal VN after holding for 4 h at 1273 K, the main phase of products is VN at 1476 K, while some vanadium nitrides transform into vanadium carbides again over 1573 K. It is found that N2 is beneficial to stimulate reduction and proceed carbonization reaction, and the reduction and nitridation reaction can occur simultaneously. The activation energy of preparing VN by one-step method is 104.005 kJ·mol−1, and the frequency factor is 470.52 at 1280–1358 K, and 150.052 kJ·mol−1 and 2.35 × 104 at 1358–1426 K, respectively.







Similar content being viewed by others
References
Zhou YB. Pan Zhihua vanadium-nitrogen alloy smelting technology. Sichuan Metall. 2012;34(1):13.
Gong DP, He MX, Luo KJ, Zeng G, Mei CY. Application of VN alloy in 400 MPa-grade reinforced bar. Iron Steel Vanadium Titan. 2001;22(1):22.
Han SH, Zhang YM, Bao SX. Effect of high calcium on roasting process of vanadium-bearing stone coal with sodium salt. Chin J Rare Met. 2013;37(6):798.
Li JH, Gao XX, Bao XQ, Cheng L, Xie JX. Wiedemann effect of Fe-Ga based magnetostrictive wires. J Chin Phys B. 2012;21(8):087501.
Wang GH, Chen YM, Zhu YK. Laboratory research on production process conditions of vanadium carbide and vanadium carbonitride. Iron Steel Vanadium Titan. 1988;2:19.
Huang ZS, Chen WL, Luo KJ, Xing JB. Development on research of vanadium nitride. Ferro-alloys. 2008;200(3):20.
Wang X, Chen BZ, Xiao WD, Peng H. Preparation technology of vanadium nitride by microwave heating. Rare Met Mater Eng. 2010;39(5):924.
Ding Y, Fu HH. Application of industrial microwave oven in producing vanadium nitride. Ferro-alloys. 2008;3:16.
Yu SS, Fu NX, Shi LK, Sui ZT. Effect of technical parameters on synthesizing vanadium carbonitride by one-step method. J Northeast Univ (Natural Science). 2006;27(2):11.
Lu ZY. Study on the preparation of high density V-N microalloy additive. Shenyang: Northeast University; 2005. 62.
Liang LK. Thermodynamic analysis of prepetition of metallic vanadium(V), vanadium carbide(VC) and vanadium nitride(VN). Iron Steel Vanadium Titan. 1999;28(3):34.
Deng L, Liu Y, Jiang ZT, Sui ZT. Synthesis of vanadium carbonitride by carbothermal reduction and nitridation method. Funct Mater. 2010;41(5):840.
Wang CZ. Metallurgical Physical Chemistry Research Method. Beijing: Metallurgical Industry Press; 2002. 391.
Coats AW, Redfern JP. Parameters from thermogravimetric data. Nature. 1964;201(4914):68.
Hu RZ, Gao SL, Zhao FQ, Shi QZ. Thermal Analysis Kinetics. Beijing: Science Press; 2008. 54.
Xu XF, Fu HH. Chemical kinetics of preparing VN from reducing and nitriding V2O5. Iron Steel Vanadium Titan. 2004;25(1):1.
Acknowledgments
This study was financially supported by the Twelfth Five-year Scientific Support Plan of the Ministry of Science and Technology of China (No. 2011BAB05B05).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, J., Yu, Y. & Xue, ZL. Non-isothermal kinetics of synthesizing vanadium nitride by one-step method. Rare Met. 34, 738–743 (2015). https://doi.org/10.1007/s12598-014-0367-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-014-0367-3