Abstract
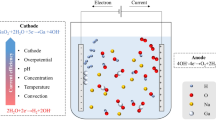
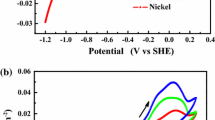
The gallium electrodeposition from alkaline solution has a very low current efficiency, the reason for which is still not quite understood. The effects of electrode materials used for gallium electrodeposition, as well as the effects of NaOH concentration and the anions concentrations in the solution, including SO4 2−, SiO3 2−, CO3 2−, AlO2 −, F−, and Cl−, on the deposition were analyzed in this study. The suitable materials of SUS316–SUS316 were suggested for the gallium electrodeposition. Based on the electrode couples, the NaOH concentration of 4 mol·L−1 for gallium electrodeposition exhibits the greatest current efficiency. Moreover, the current efficiency would decrease in the electrolyte along with the increasing concentration of the anions, except that, 0.2 mol·L−1 Cl− in the solution slightly improves the current efficiency of gallium electrodeposition. Moreover, the gallium deposited on the cathode from the solution with 0.6 mol·L−1 SiO3 2− appears tiny black in color and coarse. Meanwhile, SUS304 is shown to be not suitable to be used as cathode for the gallium electrodeposition from the alkaline solution.









Similar content being viewed by others
References
Jadvar R, McCoy BJ, Ford B, Galt J. Recovery of gallium and arsenic from GaAs wafer manufacturing slurries. Environ Prog. 1991;10(4):278.
Wu XL, Wu SK, Qin WQ, Ma XH, Niu YJ, Lai SS, Yang CR, Jiao F, Ren LY. Reductive leaching of gallium from zinc residue. Hydrometallurgy. 2012;113:195.
Nishihama S, Hirai T, Komasawa I. Separation and recovery of gallium and indium from simulated zinc refinery residue by liquid–liquid extraction. Ind Eng Chem Res. 1999;38(3):1032.
Huang Z, Yang Y, Cai J, Guo Y, Li G, Jiang T, Qiu G. Comprehensive utilization of zinc-leaching residue and concentration mechanism of gallium. J Central South Univ. 2002;33(02):133.
Kinoshita T, Akita S, Nii S, Kawaizumi F, Takahashi K. Solvent extraction of gallium with non-ionic surfactants from hydrochloric acid solution and its application to metal recovery from zinc refinery residues. Sep Purif Technol. 2004;37(2):127.
Ma X, Tan W, Wu X, Ren L. Extracting gallium and germanium from zinc-leaching residues by hot-acid leaching process. Min Metall Eng. 2012;32(02):71.
Font O, Querol X, Juan R, Casado R, Ruiz CR, Lopez-Soler A, Coca P, Garcia Pena F. Recovery of gallium and vanadium from gasification fly ash. J Hazard Mater. 2007;139(3):413.
Zeng QY. Recovery of gallium from fly ash: an experimental study. Beijing: China University of Geosciences; 2007. 13.
Bai G, Teng W, Sun Y, Wang X, Qin J. Study on acid pre-extraction process for gallium from fly ash. Appl Chem Ind. 2008;07:757.
Xu K, Deng T, Liu J, Peng W. Study on the recovery of gallium from phosphorus flue dust by leaching with spent sulfuric acid solution and precipitation. Hydrometallurgy. 2007;86(3–4):172.
Wang SJ. The research of extracting gallium from the flue dust extracted in the phosphorus industry. Kunming: Kunming University of Science and Technology; 2009. 16.
Chen YF, Yang FR, Jean JS, Tsai CY. Separation of gallium and arsenic from the wafer grinding extraction solution. J Environ Sci Health Part A. 2004;39(9):2473.
Su Y, Li G, Luo K. Research progress on extraction of metal gallium. Hydrometall China. 2003;01:9.
Gupta B, Mudhar N, Singh I. Separations and recovery of indium and gallium using bis(2,4,4-trimethylpentyl) phosphinic acid (Cyanex 272). Sep Purif Technol. 2007;57(2):294.
Katsuta S, Okai M, Yoshimoto Y, Kudo Y. Extraction of gallium(III) from hydrochloric acid solutions by trioctylammonium-based mixed ionic liquids. Anal Sci. 2012;28(10):1009.
Mahamuni SV, Wadgaonkar PP, Anuse MA. Liquid-liquid extraction and recovery of gallium(III) from acid media with 2-octylaminopyridine in chloroform: analysis of bauxite ore. J Serb Chem Soc. 2010;75(8):1099.
Liu JS, Chen H, Chen XY, Guo ZL, Hu YC, Liu CP, Sun YZ. Extraction and separation of In(III), Ga(III) and Zn(II) from sulfate solution using extraction resin. Hydrometallurgy. 2006;82(3–4):137.
Geidarov AA. Extractive recovery of gallium(iii) from acid sulfate solutions with primary amines. Russ J Appl Chem. 2008;81(12):2084.
Mihaylov I, Distin PA. Gallium solvent—extraction in hydrometallurgy: an overview. Hydrometallurgy. 1992;28(1):13.
Gupta B, Mudhar N, Begum Z, Singh I. Extraction and recovery of Ga(III) from waste material using Cyanex 923. Hydrometallurgy. 2007;87(1–2):18.
Zhao Z, Yang Y, Xiao Y, Fan Y. Recovery of gallium from Bayer liquor: a review. Hydrometallurgy. 2012;125:115.
Wang J. Production processes and uses of gallium. Sichuan Nonferrous Met. 2003;04:14.
Lu X, Wang L, Wang X, Niu X. Research progress in gallium recovery technology. Nonferrous Met. 2008;60(04):105.
Gupta B, Mudhar N, Tandon SN. Extraction and separation of gallium using Cyanex 301: its recovery from Bayer’s liquor. Ind Eng Chem Res. 2005;44(6):1922.
Kekesi T. Gallium extraction from synthetic Bayer liquors using Kelex 100-kerosene, the effect of loading and stripping conditions on selectivity. Hydrometallurgy. 2007;88(1–4):70.
Nakayama M, Egawa H. Recovery of gallium(III) from strongly alkaline media using a Kelex-100-loaded ion-exchange resin. Ind Eng Chem Res. 1997;36(10):4365.
Selvi P, Ramasami M, Samuel MHP, Sripriya R, Senthilkumar K, Adaikkalam P, Srinivasan GN. Gallium recovery from Bayer’s liquor using hydroxamic acid resin. J Appl Polym Sci. 2004;92(2):847.
Rao KS, Sarangi D, Dash PK, Chaudhury GR. Preferential extraction of gallium from Bayer liquor using ion exchange chelating resin containing hydroxamic acid functional group. J Chem Technol Biotechnol. 2003;78(5):555.
Rao KS, Chaudhury GR, Krishna PG, Das SC, Misra VN. Recovery of gallium from Bayer liquor using ion exchange technique: a case study. Miner Metall Process. 2006;23(1):22.
Riveros PA. Recovery of gallium from Bayer liquous with an amidoxime resin. Hydrometallurgy. 1990;25(1):1.
Selvi P, Ramasami M, Samuel MHP, Adaikkalam P, Srinivasan GN. Recovery of gallium from Bayer liquor using chelating resins in fixed-bed columns. Ind Eng Chem Res. 2004;43(9):2216.
Xie F, Wang J, Guo P, Wei L, Ma M, Liu P. Recovery of gallium from Bayer solution with ion exchange. Light Met. 2004;10:10.
Varadharaj A, Rao GP. Extration of gallium metal by exchange reaction between sodium amalgam and Ga(III): a cyclic voltammetric study. J Appl Electrochem. 1986;16(6):929.
Abdul Kader JAM, Varadaraj A, Srinivasan GN, Srinivasan R. Gallium as a byproduct of aluminium industries in India. Trans Indian Inst Met. 1982;35(3):276.
Chang LR, Chen YF, Yang FR. Recovering metal gallium from rich-gallium alkaline aqueous solution using electrode position process. In: Proceedings of the 8th International Symposium on East Asian Resources Recycling Technology 2005: Resources Recycling Technology, 2005: 372.
Acknowledgments
This study was financially supported by the National High Technology Research and Development Program of China (No. 2011AA060701) and the National Natural Science Foundation of China (No. 21276258).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, KK., Zhang, YF., Cao, ST. et al. Current efficiency of Ga electrodeposition under different anions concentrations. Rare Met. 35, 349–355 (2016). https://doi.org/10.1007/s12598-013-0214-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-013-0214-y