Abstract
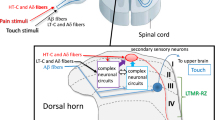
Extracellular purine nucleotides and nucleosides play important roles in the nervous system, e.g., neurotransmission, neuromodulation, chemoattraction and acute inflammation. Extracellular nucleotides act through ATP receptors (P2 receptors). P2 receptors are classified into two families: the P2X receptors are ionotropic ligand-gated ion channels and the P2Y receptors are metabotropic G-protein-coupled receptors. Currently, seven P2X receptors (P2X1–7) and eight P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14) are recognized. In the sensory nervous system, ATP is suggested to be one of first mediators of tissue damage, which activates primary afferents. Nerve injury often leads to neuropathic pain, such as mechanical allodynia and painful responses to normally innocuous stimuli. Peripheral nerve injury induces the upregulation of molecules in activated microglia in the spinal cord. Microglia in the spinal cord may play an important role in the development and maintenance of neuropathic pain. A prominent signaling pathway in the development of neuropathic pain involves ATP acting on microglial purinergic receptors. This review focuses on the expression of P2X and P2Y receptors mRNAs in the pain transmission pathway, i.e., in the dorsal root ganglion (DRG) and spinal cord. Furthermore, we suggest that the multiple microglial P2Y receptors activated by peripheral nerve injury may play a key role in the development of neuropathic pain.


Similar content being viewed by others
References
Abbadie C, Bhangoo S, De Koninck Y et al (2009) Chemokines and pain mechanisms. Brain Res Rev 60:125–134
Agteresch HJ, Dagnelie PC, van den Berg JW, Wilson JH (1999) Adenosine triphosphate: established and potential clinical applications. Drugs 58:211–232
Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284
Beggs S, Salter MW (2007) Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun 21:624–633
Bianco F, Fumagalli M, Pravettoni E et al (2005) Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev 48:144–156
Burnstock G (2000) P2X receptors in sensory neurones. Br J Anaesth 84:476–488
Burnstock G (2006) Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci 27:166–176
Burnstock G (2007) Purine and pyrimidine receptors. Cell Mol Life Sci 64:1471–1483
Burnstock G, Krugel U, Abbracchio MP, Illes P (2011) Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol 95:229–274
Chen CC, Akopian AN, Sivilotti L et al (1995) A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377:428–431
Chessell IP, Hatcher JP, Bountra C et al (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114:386–396
Cockayne DA, Dunn PM, Zhong Y et al (2005) P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol 567:621–639
Collier HO, James GW, Schneider C (1966) Antagonism by aspirin and fenamates of bronchoconstriction and nociception induced by adenosine-5′-triphosphate. Nature 212:411–412
Collo G, North RA, Kawashima E et al (1996) Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci 16:2495–2507
Corriden R, Insel PA (2010) Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal 3:re1
Eriksson NP, Persson JK, Svensson M et al (1993) A quantitative analysis of the microglial cell reaction in central primary sensory projection territories following peripheral nerve injury in the adult rat. Exp Brain Res 96:19–27
Fischer W, Krugel U (2007) P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem 14:2429–2455
Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP (2006) Pharmacology of P2X channels. Pflugers Arch 452:513–537
Grant K, Knowles J, Dawas K et al (2007) Mechanisms of endothelin 1-stimulated proliferation in colorectal cancer cell lines. Br J Surg 94:106–112
Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD (2007) Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol 72:1447–1456
Haynes SE, Hollopeter G, Yang G et al (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9:1512–1519
He WJ, Cui J, Du L et al (2012) Spinal P2X(7) receptor mediates microglia activation-induced neuropathic pain in the sciatic nerve injury rat model. Behav Brain Res 226:163–170
Jahr CE, Jessell TM (1983) ATP excites a subpopulation of rat dorsal horn neurones. Nature 304:730–733
Ji RR, Gereau RW, Malcangio M, Strichartz GR (2009) MAP kinase and pain. Brain Res Rev 60:135–148
Jin SX, Zhuang ZY, Woolf CJ, Ji RR (2003) p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci 23:4017–4022
Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413:203–210
Kobayashi K, Fukuoka T, Yamanaka H et al (2005) Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol 481:377–390
Kobayashi K, Fukuoka T, Yamanaka H et al (2006) Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol 498:443–454
Kobayashi K, Yamanaka H, Fukuoka T et al (2008) P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci 28:2892–2902
Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, Noguchi K (2011) Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci Lett 504:57–61
Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K (2012) Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia 60:1529–1539
Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K et al (2007) UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446:1091–1095
Lewis C, Neidhart S, Holy C et al (1995) Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377:432–435
Light AR, Wu Y, Hughen RW, Guthrie PB (2006) Purinergic receptors activating rapid intracellular Ca increases in microglia. Neuron Glia Biol 2:125–138
McGaraughty S, Chu KL, Namovic MT et al (2007) P2X7-related modulation of pathological nociception in rats. Neuroscience 146:1817–1828
McLarnon JG (2005) Purinergic mediated changes in Ca2 + mobilization and functional responses in microglia: effects of low levels of ATP. J Neurosci Res 81:349–356
Milligan ED, Sloane EM, Watkins LR (2008) Glia in pathological pain: a role for fractalkine. J Neuroimmunol 198:113–120
Moriyama T, Iida T, Kobayashi K et al (2003) Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci 23:6058–6062
Nicke A (2008) Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem Biophys Res Commun 377:803–808
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Ohsawa K, Irino Y, Nakamura Y et al (2007) Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia 55:604–616
Petruska JC, Cooper BY, Gu JG, Rau KK, Johnson RD (2000) Distribution of P2X1, P2X2, and P2X3 receptor subunits in rat primary afferents: relation to population markers and specific cell types. J Chem Neuroanat 20:141–162
Praetorius HA, Leipziger J (2009) ATP release from non-excitable cells. Purinergic Signal 5:433–446
Ransohoff RM, Perry VH (2009) Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 27:119–145
Scholz J, Woolf CJ (2002) Can we conquer pain? Nat Neurosci 5(Suppl):1062–1067
Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10:1361–1368
Schomberg D, Olson JK (2012) Immune responses of microglia in the spinal cord: contribution to pain states. Exp Neurol 234:262–270
Shieh CC, Jarvis MF, Lee CH, Perner RJ (2006) P2X receptor ligands and pain. Expert Opin Ther Pat 16:1113–1127
Smith HS (2010) Activated microglia in nociception. Pain Physician 13:295–304
Souslova V, Cesare P, Ding Y et al (2000) Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 407:1015–1017
Torres GE, Egan TM, Voigt MM (1999) Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J Biol Chem 274:6653–6659
Tozaki-Saitoh H, Tsuda M, Miyata H et al (2008) P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci 28:4949–4956
Tsuda M, Shigemoto-Mogami Y, Koizumi S et al (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424:778–783
Tsuda M, Inoue K, Salter MW (2005) Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci 28:101–107
Visentin S, Nuccio CD, Bellenchi GC (2006) Different patterns of Ca(2+) signals are induced by low compared to high concentrations of P2Y agonists in microglia. Purinergic Signal 2:605–617
von Kugelgen I (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110:415–432
Vulchanova L, Riedl MS, Shuster SJ et al (1997) Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology 36:1229–1242
Woolf CJ, Salter MW (2000) Neuronal plasticity: increasing the gain in pain. Science 288:1765–1769
Wu LJ, Vadakkan KI, Zhuo M (2007) ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia 55:810–821
Zhuo M, Wu G, Wu LJ (2011) Neuronal and microglial mechanisms of neuropathic pain. Mol Brain 4:31
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research and the Research Basis Formation Supporting Project for Private University, both from the Japanese Ministry of Education, Science, and Grants-in-Aid for Researchers Hyogo College of Medicine 2009. We thank D.A. Thomas for correcting the English usage.
Conflict of interest
The authors declare no conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, K., Yamanaka, H. & Noguchi, K. Expression of ATP receptors in the rat dorsal root ganglion and spinal cord. Anat Sci Int 88, 10–16 (2013). https://doi.org/10.1007/s12565-012-0163-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-012-0163-9