Abstract
Brain-resident macrophages (microglia) are key cellular elements in the preservation of tissue integrity. On the other hand, they can also contribute to the development of pathological events by causing an extensive and inappropriate inflammatory response. A growing number of reports indicate the involvement of nucleotides in the control of microglial functions. With this study on P2Y receptors in rat microglia, we want to contribute to the definition of their expression profile and to the characterisation of their signalling mechanisms leading to Ca2+ movements. Endogenous nucleotides, when applied at a concentration of 100 μM, elicited robust Ca2+ transients, thanks to a panel of metabotropic receptors comprising mainly P2Y2, P2Y6 and P2Y12 subtypes. The involvement of P2Y12 receptors in Ca2+ responses induced by adenine nucleotides was confirmed by the pharmacological and pertussis toxin sensitivity of the response induced by adenosine diphosphate (ADP). Beside the G protein involved, Gi and Gq respectively, adenine and uracil nucleotides differed also for induction by the latter of a capacitative Ca2+ plateau. Moreover, when applied at low (sub-micromolar) concentrations with a long-lasting challenge, uracil nucleotides elicited oscillatory Ca2+ changes with low frequency of occurrence (≤ 1 min−), sometimes superimposed to an extracellular Ca2+-dependent sustained Ca2+ rise. We conclude that different patterns of Ca2+ transients are induced by low (i.e., oscillatory Ca2+ activity) compared to high (i.e., fast release followed by sustained raise) concentrations of nucleotides, which can suggest different roles played by receptor stimulation depending not only on the type but also on the concentration of nucleotides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Distributed in the central nervous system (CNS) parenchyma in a ramified morphology, microglial cells play a continuous “resting activity” of surveillance. Signals not well identified as yet seem to be required to keep microglia in such a quiescent but alert state. Similarly, signals resulting from an ongoing acute insult or from a chronic degenerative condition drive toward or maintain microglia in a state of cell activation. Synthesis of pro- and anti-inflammatory cytokines, reactive oxygen species, nitric oxide, prostaglandins, expression of major histocompatibility complex (MHC) molecules and re-arrangement of the ion channel profile are only some of the changes acquired by activated microglial cells [1–3]. In principle, these newly acquired functional capabilities would tend to favour protection and recovery of homeostasis, but in some instances, control on the overall inflammatory response is lost and an exacerbation of the pathology takes place. Participation in the pathogenesis of virtually all acute and chronic diseases has been suggested for microglia. The dichotomy of microglia between guardian and potentially promoter of recovery on one hand and pathogenic cellular entity on the other hand has drawn interest to the search for signals driving microglia to beneficial capabilities and the control of pro-inflammatory and potentially harmful activities [4, 5].
Adenosine triphosphate (ATP) and other nucleotides have been extensively described as multi-functional agents acting on neuronal and glial cells. ATP acts as a fast synaptic neurotransmitter by binding to post-synaptic P2X receptors in a variety of neuronal cells, in keeping with their wide distribution predominantly on brain neurons [6]. ATP also acts on postsynaptic P2Y receptors, modulating voltage-dependent K+ and Ca2+ currents [7, 8]. P2Y receptors were also described to interfere with N-methyl-D-aspartate (NMDA) but not with alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) glutamate receptors, probably by inducing phosphorylation of the receptor protein [9]. ATP and analogues acting on P2Y receptors regulate the release of neurotransmitters such as acetylcholine, noradrenaline, dopamine, serotonin and glutamate (6 for references) in concert with adenosine.
Also, all glial cell types have been described to react to ATP and other nucleotides. ATP and analogues were shown to induce astrocyte proliferation, stellation and increase of glial fibrillary acidic protein (GFAP) expression, all symptoms of astrogliosis [10–12], by stimulating P2Y receptors. Ca2+ wave propagation in mechanically or pharmacologically stimulated cultured astrocytes are known to depend on the release of ATP and on its effect on adjacent cells [13]. ATP released from stimulated dorsal root ganglia (DRG) cells was also described to modulate the differentiation and proliferation of Schwann cells in a in vitro co-culture system [14]. Recently, we identified ATP and adenosine diphosphate (ADP) as agents able to control differentiation and promote migration of oligodendrocyte progenitors by activation of P2Y1 receptors [15]. A number of different effects of nucleotides on microglial cells have also been reported. In microglia, great attention has been dedicated to P2X ionotropic receptors, and thanks to this, their profile of expression and a set of functions clearly linked to each subtype has been delineated. P2X7 receptors, first described for their pro-inflammatory capabilities due to the induction of interleukin (IL)-1β and tumour necrosis factor (TNF)-α release [16, 17], as well as for their cytotoxicity due to the opening of a large conductance pore [18], are now considered also as involved in the fine control of microglial proliferation (Bianco et. al., this issue). A second ionotropic ATP receptor that we tentatively proposed in microglia as the P2X4 subtype on the basis of kinetic and pharmacological properties [19] was then clearly identified in this cell type, and its role in the induction of tactile peripheral allodynia following nerve injury was proposed [20]. A number of studies have addressed the capabilities of nucleotides to mobilise Ca2 and to induce the opening of store-operated Ca2+ channels by acting on microglial P2Y receptors [21, 22]. Chemotactic activity induced by ATP was described to involve P2Y12 receptors [23] while the release of pro- and anti-inflammatory cytokines was shown to be under the control of as yet unidentified P2Y receptors [24–26].
Glial cells are not only targets of nucleotide signalling, they also are more generally responsible of shaping the nucleotide signalling system, being also sources of nucleotides and in charge of their degradation. Regardless of the mechanisms utilised—vesicular, mediated by ATP-binding cassette (ABC) transportes or through connexin emichannels—during Ca2+ waves propagation, astrocytes release ATP, which acts as an extracellular messenger in homo-typical (i.e., astrocyte to astrocyte) and hetero-typical (i.e., astrocyte to microglia) intercellular communication [13, 27]. When challenged with the pro-inflammatory factor lipopolysaccharide (LPS), microglia was described to release ATP, which, by acting as an autocrine factor, contributed to processing and release of cytokines IL-β1 and IL-10 [16, 25]. Finally, astrocytes and microglial cells are deputed to the degradation of extracellular nucleotides in virtue of the expression of ecto-enzymes ecto-ATPase, ecto-apyrase and ecto-5′-nucleotidase [28]. Interestingly, increase of expression of these enzymes after transient forebrain ischaemia in the rat was shown to involve mainly microglia [29], which makes these cells capable of reacting to the increase of nucleotide release in the attempt to avoid excessive nucleotide stimulation.
It is known that high doses of nucleotides released by tissue undergoing stress, cell damage or abnormal death might act as “danger signals” and cause release of proinflammatory cytokines (IL-1β, TNF-α, IL-6), prostaglandins and reactive oxygen species [30] from immune cells. On the other hand, it was recently suggested that at lower doses, nucleotides might play a regulatory role on inflammatory reactions aimed at the prevention of tissue damage [31].
In this study, we focused on P2 metabotropic receptors in rat microglial cells, and by molecular [reverse transcription polymerase chain reaction (RT-PCR)] and functional (Ca2+ video imaging) approaches, we characterised expression profile, functional capability and some aspects of the pathways involved in receptor activation. By comparing the effects induced by low versus high concentrations of metabotropic agonists, we then aimed to depict dependence on the availability of nucleotides of cytoplasmic Ca2+ signalling.
Materials and methods
Cell culture
Microglial cells were obtained from the cerebral cortex of 1- to 2-day-old rats [32]. All experiments were carried out in accordance with directives of the Council of the European Communities N. 86/609/CEE. Briefly, after pups were rendered hypothermic on an ice-cold surface, they were decapitated. The cerebral cortex was dissected out, and meninges were removed. The tissue was dissociated by a two-step procedure comprising enzymatic digestion followed by mechanical dissociation. Mixed primary cultures were grown on poly-L-lysine-coated culture flasks for 7–10 days in Basal Medium Eagle (BME) supplemented with 10% endotoxin-free foetal calf serum (FCS), 2 mM glutamine and 100 µg ml−1 gentamicin (37°C, 5% CO2). After mild shaking, microglial cells were harvested and plated on un-coated glass coverslips sealed on homemade silicon wells at a density of 15 × 104 cells/cm2. To further improve the purity of microglial cultures, non-adhering cells were removed after 20 min by changing the medium.
RT-PCR
Microglia cells obtained from rat brain at postnatal day 2 were used to extract total ribonucleic acid (RNA) using Trizol reagent according with the data sheet. Two micrograms of total RNA were digested for 30 min with Dnase (Invitrogen) and retro-transcribed using the Super-Script synthesis system (Life Technologies). Specific oligonucleotides with similar annealing temperatures were designed to amplify the following purinergic receptors: P2Y1 (NM012800.1), P2Y2 (NM017255.1), P2Y4 (NM031680.1), P2Y6 (NM057124.1), P2Y12 (NM022800.1), P2Y13 (NM001002853.1) and P2Y14 (NM133577.1). β-actin (NM031144) was always amplified as control. Samples were analysed on agarose gel containing ethidium bromide.
The following primers were used:
P2Y1: forward: 5′-AACAGTACAATCGCCTCGAC-3′; reverse: 5′-TTCGCAGGTACTCATCTGAC-3′. Band size of 559 bp.
P2Y2: forward: 5′AACGGACGTGAGCATCCAA3′; reverse: 5′-AGGTGTCGTGGCAGGTGATT-3′. Band size of 639 bp.
P2Y4: forward: 5′-CCATCCTGTGCCATGACACT-3′; reverse: 5′-CATAGCTGCTGGAGCTGGTT-3′. Band size of 418 bp.
P2Y6: forward: 5′-CGGCAGCATCCTGTTCCTCA-3′; reverse 5′-TGGAGCGCACAGCCAAGTAG-3′. Band size of 470 bp.
P2Y12: forward: 5′-GGTGTGCCAAGTCACTTCAG; reverse 5′-ATCAAGGCAGGCGTTCAAGG-3′. Band size of 598 bp.
P2Y13: forward: 5′-GATGACACAGCTGCTGTTCC-3′; reverse: 5′-TGACTGCTGTGGTGCTCATC-3′. Band size of 915 bp.
P2Y14: forward: 5′-GCACAAGGCGTCTAACTATGTC-3′; reverse: 5′-CCACATGCCACTTCTCCAT TCG-3′. Band size of 692 bp.
β-Actin: forward: 5′-ACCTTCAACACCCCAGCCAT GTAC G-3′; reverse: 5′-CTGATCCACATCTGCTGG AAGGTGG-3′. Band size of 698 bp.
Intracellular Ca2+ recording
To record intracellular Ca2+ we employed the video-imaging technique with the Ca2+-sensitive probe Fura-2. Acetoxymethyl Fura-2 (Fura-2-AM; Molecular Probes, Leiden, The Netherlands) was dissolved in a solution containing pluronic acid and dimethylsulfoxide (DMSO) (1:4 ratio in weight) and then sonicated for 5 min. Microglial cultures were exposed to a solution containing 2.5 µM Fura-2-AM for 50 min at room temperature to allow cell loading. To achieve a better hydrolysis of Fura-2-AM, the cultures were recorded 30 min after washing of the dye. Silicon wells were removed, and the glass coverslips were placed in a recording chamber on the stage of an Axiovert 35 inverted microscope (Zeiss, Milan, Italy). Fura-2-loaded cells were exposed every 1–5 s to the excitation wavelengths 340 and 380 nm by means of a monochromator (Polychrome II, T.I.L.L. Photonics, Planegg, Germany). The emission light at 510 nm was collected by a digital camera (Sensicam, PCO, Kelheim, Germany) and recorded on the hard disk of a PC computer. Recording and analysis of data were made possible by the Imaging Workbench 5.2 software package (Indec BioSystems, Santa Clara, CA, USA). Ratio values were converted to free Ca2+ concentrations by utilising an established equation [33], with Rmin = 0.28, Rmax = 6.45, β = 4.5.
Unless otherwise stated, the bulk solution had the following composition (mM): 140 NaCl, 5 KCl, 2.5 CaCl2, 1 MgCl2, 10 D-glucose, 10 HEPES/NaOH (room temperature, pH 7.4, 290 mosmol l−1). Ca2+-free solutions were made by replacing Ca2+ with an equal amount of Mg2+. In parallel with a perfusion system allowing the change of the bulk solution of the bath, a local perfusion system was used (Rapid Solution Changer: RSC 100; Biologic, Grenoble, France). It consisted of a set of reservoirs containing different solutions connected to polyethylene tubings with an internal diameter of 300 µm glued together in a parallel array from which the flow of solution was made possible by operating on solenoid valves. Amplitudes of Ca2+ signals were calculated as Ca2+ increase from the pre-application Ca2+ level. Peak amplitudes were calculated on single cells, and their averages were calculated pooling similar experiments (from a minimum of two experiments for each condition). Not to underestimate an eventual effect on sub-populations of cells, we thought it useful to represent the data as frequency distributions of the experimental values measured in the single cells. The results obtained with uridine 5′-diphosphate (UDP) were not statistically different from those obtained with uridine 5′-triphosphate (UTP), which we showed to represent uracil nucleotides in all figures following Figure 2.
Adenosine triphosphate (ATP), adenosine diphosphate ((ADP), uridine 5′-triphosphate (UTP) and uridine 5′-diphosphate (UDP) but not UDP-glucose (P2Y14 agonist) induce intracellular Ca2+ release in microglia. Microglial cells were challenged with a panel of naturally occurring nucleotides for 20 s at a concentration of 100 µM. With the exception of UDP-glucose, nucleotide application caused in virtually all cells challenged a fast Ca2+ rise followed by a variable phase of recovery. The exemplifying traces depict the fact that uracil nucleotides (UTP and UDP) more consistently induced a Ca2+ plateau following the initial peak
Results
mRNA expression of P2Y receptors
In order to understand which P2Y receptors are expressed in primary microglial cells, we performed RT-PCR analysis on purified messenger ribonucleic acid (mRNA) obtained from primary microglial cells. Specific amplification profiles are shown in Figure 1. High mRNA expression was detected for P2Y2, P2Y6, P2Y12 and P2Y14. The weak band detected for P2Y1 and P2Y4 could not exclude their presence. Negative control (PCR without Retro transcription) was always performed (data not shown).
Expression pattern of P2Y receptors analysed by reverse transcription polymerase chain reaction (RT-PCR). (a) In microglial cells, we found the expression of P2Y2, P2Y6, P2Y12 and P2Y14 receptors. Moreover, a weak amplification of the P2Y1 and P2Y4 receptors was detected. Ethidium-bromide-stained agarose gel shows RT-PCR products generated from total ribonucleic acid (RNA) of microglia. (b) In control samples, complementary deoxyribonucleic acid (cDNA) was reverse transcribed and amplified for β-actin. All samples were preincubated with DNAse before retro-transcription
Effects of endogenous nucleotides on Ca2+
To contribute to the determination of P2Y receptors functionally active in our rat microglial cell cultures, we utilised a number of endogenous nucleotides (ATP, ADP, UTP, UDP, UDP-glucose). When briefly applied at a high concentration (100 µM for 20 s), all of them, with the exception of UDP-glucose, were able to induce a fast Ca2+ rise followed by a slower phase of recovery in virtually all microglial cells (Figure 2). The lack of P2Y14 protein (to be tested when an appropriately validated antibody is available) or the expression of a protein not functionally coupled to Ca2+ release [15] can explain the apparent incongruence between PCR and Ca2+ data.
The speed of rise and average Ca2+ amplitude were generally unaffected by removal of Ca2+, with the exception of a slight decrease of the Ca2+ concentration reached when ATP was used (data not shown). Molecular and functional data are compatible with a P2Y receptor profile in which the P2Y12 is responsible for adenine nucleotide-induced Ca2+ signals and P2Y2 and P2Y6 for UTP- and UDP-induced Ca2+ signals. To confirm this description, especially the role of P2Y12 in Ca2+ signalling, further studies testing the pharmacological and pertussis toxin sensitivity of the ADP response were carried out.
Pharmacological blockade of P2Y receptors
For a pharmacological characterisation of P2Y receptors, we utilised PPADS and MRS2179. PPADS is a non-selective but non-universal P2 receptor antagonist. It efficiently inhibits P2X receptors, but it discriminates between ADP and UTP responses on P2Y receptors. Hence, we used it to confirm the specificity of the effect of ADP and uracil nucleotides. At a concentration of 100 µM, PPADS completely inhibited the response to ADP and UTP in 78% and 18% of the cells (Figure 3a, c), respectively, and only partially affected the responses induced in the remaining cells.
Ca2+ release induced by nucleotides is differently affected by pharmacological inhibitors. Ca2+ transients induced by adenosine diphosphate (ADP) and uridine 5′-triphosphate (UTP) in the absence of Ca2+ were recorded in the absence or during the application of P2 antagonists (100 µM, with 2 min of pre-treatment). Each panel shows frequency distribution of amplitudes of the Ca2+ transients recorded in the absence and presence of the antagonist. (a), (c) PPADS more efficiently inhibits the ADP-compared with UTP-induced Ca2+ responses (n = 64–103). (b), (d) As expected, the P2Y1 antagonist MRS2179 does not affect the response to UTP but it does not inhibit even that to ADP, outlining the presence of ADP-sensitive receptors other than P2Y1 (n = 59–164)
MRS2179 can be reliably utilised to detect involvement of P2Y1 receptors, as we recently confirmed in oligodendrocyte progenitors [15]. MRS2179, up to a concentration of 100 µM, did not inhibit Ca2+ responses induced by UTP, as expected considering that UTP acts on receptors other than P2Y1 (Figure 3d). However, it was unable to inhibit even ADP-induced responses (Figure 3b), so confirming that P2Y1 receptor activation is not appreciably involved in the induction of Ca2+ movements by ADP in rat microglia. On the other hand, this observation is in keeping with a role played by the P2Y12 subtype in ADP-induced Ca2+ responses.
G protein involvement
As mentioned above, P2Y receptors act by preferentially binding and activating specific hetero-trimeric G proteins. Only 10% of microglial cells treated overnight with PTx were able to show a Ca2+ response when challenged with ADP (Figure 4a) while 70% of cells maintained a Ca2+ response to UTP (Figure 4c) though lacking the capacitative plateau (Figure 4d). PTx-treated cells still responding to ADP showed a reduced Ca2+ signal (236 ± 6 nM and 134 ± 13 nM in control and treated cultures, respectively) while cells still responding to UTP after PTx-treatment maintained a similar Ca2+ signal (190 ± 8 nM versus 172 ± 6 nM). Involvement of PTx-sensitive Gi in the mechanism leading to Ca2+ rise by ADP further supports the idea that activation of P2Y12 receptors is responsible for ADP-induced Ca2+ movements in microglia. When ATP was tested in PTx-treated cultures, it elicited a Ca2+ transient in only a fraction of cells in the absence of Ca2+, and it caused small Ca2+ rises in most cells when Ca2+ was present, depicting Ca2+ influx through ionotropic receptors (Figure 4b).
Pertussis toxin completely inhibits Ca2+ release induced by adenine nucleotides but not by uridine 5′-triphosphate (UTP). (a), (c) Ca2+ transients induced by nucleotides (100 µM for 20 s in the absence of Ca2+) were recorded from control microglial cultures and cultures treated overnight with pertussis toxin (PTx) 50 ng/ml. More than 90% of PTx-treated cells were unable to respond to adenosine diphophate (ADP) (n=107–271) while only 30% did not respond to UTP (n=98–289). (b) Exemplifying Ca2+ traces recorded from PTxtreated cultures show the lack of response to ADP, the rare response to ATP in the absence of Ca2+ and the lower but consistent Ca2+ transients triggered by ATP in the presence of Ca2+, depicting the lack of sensitivity to PTx of the ATP-induced ionotropic response. (d) Exemplifying Ca2+ traces recorded from PTx-treated cultures showing UTP-induced Ca2+ signals with amplitudes comparable with those obtained in control conditions but without a sustained plateau
Capacitative plateau
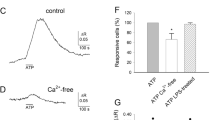
According to the above data, a striking difference among the signals triggered by adenine and uracil nucleotides is the main G protein involved. A second difference is the much greater capability of uracil nucleotides to induce, after the rapid Ca2+ rise, a sustained Ca2+ plateau sometimes appearing as a second and clearly distinguishable phenomenon (Figure 2). According to extracellular Ca2+ dependence (data not shown) and blockade by SKF96395 (Figure 5a), it was identified as the capacitative plateau described in a number of cell types. According to the canonical description, its induction is due to the opening of store-operated Ca2+ (SOC) channels on the plasma membrane, following (through a not as yet clearly determined mechanism) Ca2+ depletion in the endoplasmic reticulum. However, this latter mechanism by itself can unlikely account for the induction of SOC opening in microglia since ADP and UTP share the same endoplasmic reticulum (ER) Ca2+ stores (Figure 5c, d), as depicted by “cross-desensitisation” experiments, and at the dose used both agents are able to efficiently induce TER Ca2+ depletion. A second mechanism triggered by uracil nucleotides but not by ADP and acting in parallel to store depletion must be involved.
Nucleotide-induced capacitative Ca2+ plateau. (a), (b) Uridine 5′-triphosphate (UTP)-induced Ca2+ transients were recorded from control cells (left panel) and cells treated with the store-operated Ca2+ (SOC) channel blocker SKF96395 (30 µM). The Ca2+ plateau induced by UTP was abrogated by SKF96395, depicting the nature of the plateau phase, while the Ca2+ peak (due to Ca2+ release) was unaffected (n = 81). (c), (d) Cross-desensitisation of he adenosine diphosphate (ADP)- and UTP-induced responses. In the absence of Ca2+, a series of challenges with the same nucleotide (100 µM for 20 s) was followed by a single challenge with a different nucleotide. Both nucleotides (ADP and UTP) very promptly caused Ca2+ depletion since in this experiment, after two challenges Ca2+, release was abolished. Cross-desensitisation of responses elicited by the two nucleotides depicts the fact that the receptors activated by the different nucleotides share the same Ca2+ stores (n=116–186)
Ca2+ oscillations induced by low doses of nucleotides
In the attempt to describe P2Y signalling in microglia, we have so far depicted some peculiar features of adenine compared with uracil nucleotides, which can cause different functional roles of P2Y subtypes and of their endogenous agonists. Then we wanted to investigate the possibility that differences in the availability of nucleotides for receptor occupancy and activation could cause qualitatively different patterns of signalling and, in turn, functional outcomes. A long-lasting challenge with nucleotides at a concentration of 100 µM (which is a high dose for metabotropic receptors) caused an initial Ca2+ peak followed by a maintained Ca2+ rise only slowly declining in the continuous presence of the agonist (Figure 6a,b). On the contrary, when using sub-micromolar concentrations (100–300 nM), a fraction of cells reacted to the challenge with a first Ca2+ peak. More importantly, a maintained Ca2+ activity was rarely detected when using ADP (Figure 6c) while a low-frequency (≤1 min−1) oscillatory Ca2+ activity was detected when cells were challenged with UTP (Figure 6d). In a fraction of cells showing oscillatory Ca2+ movements, a slow Ca2+ rise overlapped Ca2+ oscillations (Figure 7a). When the extracellular Ca2+ dependence was tested, the slow rise of Ca2+ disappeared in the absence of extracellular Ca2+ while the fraction of cells showing oscillatory activity did not change (Figure 7b). These observations are compatible with Ca2+ movements from intracellular compartments and Ca2+ entrance as the mechanisms causing oscillatory activity and slow Ca2+ rise, respectively. Moreover, in the time span evaluated (5–10 min), the frequency of oscillations seemed to be unaffected by Ca2+ removal, so depicting a mechanism probably independent from extracellular Ca2+. The most probable source for Ca2+ oscillations is the ER. To test this hypothesis, ER were depleted by repeated challenges with high doses of nucleotides or by pre-treatment with the ER Ca2+-ATPase inhibitor thapsigargin (Figure 8a, b). In neither of the cases could a subsequent application of a low dose of UTP trigger Ca2+ oscillations, so confirming that the ER was the source of Ca2+.
Different patterns of Ca2+ signals are induced by high compared to low concentrations of nucleotides. (a), (b) Long-lasting challenges with a high dose of nucleotides [adenosine diphosphate (ADP) and uridine 5′-triphosphate (UTP): 100 µM for (2–5 min) were applied to microglial cells. The exemplifying Ca2+ traces depict the fast Ca2+ increase followed by a long-lasting and slowly decaying Ca2+ plateau caused by both nucleotides (left panel: ADP; right panel: UTP). C, D Long-lasting challenges with low doses of nucleotides (ADP and UTP: 300 nM for 5–10 min) were applied to microglial cells. As depicted by the exemplifying Ca2+ traces, ADP triggered a single Ca2+ peak (c) while UTP caused a long-lasting oscillatory Ca2+ activity (d)
Ca2+ signal induced by low doses of uridine 5′-triphosphate (UTP) only partially depend on the presence of extracellular Ca2+. (a) When challenged with a low dose of UTP, microglial cells reacted with Ca2+ signals with the following patterns: a single Ca2+ peak, an oscillatory Ca2+ activity and an oscillatory Ca2+ activity superimposed to a slowly raising Ca2+ plateau. (b) Different populations of microglial cells were challenged with UTP 300 nM in the presence or absence of Ca2+, and the percentage of cells reacting with the different patterns of Ca2+ signals was calculated (n = 198–247). The absence of Ca2+ did not affect the percentage of occurrence of the single peak nor of the oscillatory activity while completely abrogated the slow rise of Ca2+
Oscillatory Ca2+ activity is abrogated by depleting Ca2+ from the endothelial reticulum (ER) stores. (a), (b) Ca2+ depletion from the ER stores was achieved by a repetitive receptor activation (a) or by applying the sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) inhibitor thapsigargin (b) in the absence of Ca2+. Following a series of applications of uridine 5′-triphosphate (UTP) 100 µM or a 2-min-long treatment with thapsigargin (100 nM), 300 nM UTP was applied to microglial cells. The exemplifying Ca2+ traces show the absence of oscillatory activity following endothelial reticulum (ER) Ca2+ depletion (n=60–94)
Potential regulatory mechanisms of Ca2+ oscillations
Once we ascertained the dual nature of the effect of low doses of uracil nucleotides and the ER as the source of Ca2+ oscillations, we wanted to investigate their possible mechanisms of regulation. According to the previous literature on Ca2+ oscillations in other cell types, two mechanisms might also apply to microglial cells: an inhibitory mechanisms triggered by capacitative Ca2+ influx [34] and a facilitating role played by mitochondria [35]. When a challenge with a low dose of UTP followed previous application of a high dose of the same nucleotide, to induce Ca2+ influx, Ca2+ oscillations were not affected (Figure 9a). Neither the percentage of cells showing Ca2+ oscillations (59 ± 6% and 51 ± 12% of the cells in control and after UTP 100 µM, respectively) nor the frequency of Ca2+ oscillations (0.37 ± 0.04 and 0.49 ± 0.06 min−1, in control and after UTP 100 µM, respectively) significantly changed after a pre-application of 100 µM UTP. In a further attempt to detect such regulatory mechanism, we used the calcineurin inhibitor cyclosporin A since capacitative Ca2+ influx was shown to inhibit Ca2+ oscillation by a mechanism involving calcineurin [34]. Also in this case, neither the percentage of cells showing Ca2+ oscillations (56 ± 6%) nor the frequency of Ca2+ oscillations (0.45 ± 0.06 min−1) were affected. We then focused on mitochondria as possible regulators of oscillations. To this aim, we evaluated the effect on oscillatory activity of the mitochondrial uncoupler carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP). After a 2- to 4-min-long pre-treatment with this agent, to cause mitochondrial depolarisation, the percentage of cells showing Ca2+ oscillations dropped to 14%, suggesting a possible role played by mitochondria in the establishment of Ca2+ oscillations.
Oscillatory Ca2+ activity is regulated by mitochondrial “energy charge” but not by a previous activation of the capacitative influx. (a) To point out the eventual regulatory role on Ca2+ oscillations of a high dose of uridine 5′-triphosphate (UTP), an application of 100 µM of the nucleotide was applied prior to an application of 300 nM of the same nucleotide. As depicted by the exemplifying Ca2+ signals, Ca2+ oscillatory activity did not differ from that recorded from non-pre-treated cells (n = 64). (b) To evaluate the regulatory role of calcineurin activated by a previous Ca2+ influx through capacitative channels, an application of 100 µM UTP was followed by an application of 300 nM of the same nucleotide in the presence of the calcineurin inhibitor cyclosporin-A (1 µM). Also in this case, the oscillatory Ca2+ activity was not affected (n = 90). (c) To evaluate the regulatory role of mitochondrial activity on oscillatory Ca2+ activity, microglial cells were treated with the mitochondrial uncoupler carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) (2 µM for 3 min) prior to 300 nM UTP. As shown by the exemplifying Ca2+ traces, FCCP pre-treatment completely abrogated UTP-induced oscillatory Ca2+ activity, suggesting the involvement of mitochondria in the regulation of the oscillatory Ca2+ activity (n = 273)
Discussion
With this study, we wanted to contribute to the understanding of metabotropic P2 receptor signalling in microglia by offering our description of P2Y receptor expression pattern, by depicting some of the features of their signalling and by focusing on the role of agonist availability on the pattern of the Ca2+ signals.
Molecular and functional expression profile of P2Y receptors
Since the first electrophysiological reports on microglial cells, nucleotides are known to stimulate both ionotropic and metabotropic receptors. According to these first studies, activation of the former caused inward currents and depolarisation of membrane potential and activation of the latter induced a K+ outward current and Ca2+ release [36–38]. Since then, several insights have been added to the picture of P2 receptor signalling in microglial cells, contributing to delineate a complex array of possible functions. Nevertheless, focused studies on receptor expression, especially of P2Y metabotropic receptors, were missing. Only recently an exhaustive picture of P2 receptor expression in microglia was published, thanks to the work by Verderio and collaborators [39]. Our description of the P2Y receptor profile diverges from the one proposed in the above article for the presence of functional P2Y1 and P2Y14 receptors, which we did not detect in our study. P2Y1 and P2Y12 receptors were found to co-exist in platelets and both to concur to the control of platelet aggregation [40]. Based on the insensitivity to MRS2179 and on the PTx-induced inhibition of the Ca2+ signal induced by ADP, we depicted P2Y12 rather than P2Y1 receptors as the ones responsible for the Ca2+ transients elicited by ADP stimulation. However, we cannot exclude a slight expression of the mRNA coding the P2Y1 receptor. Moreover, as already suggested [39], we depicted the capability of P2Y12 receptors to move Ca2+ by a mechanism that we describe as PTx sensitive. These aspects of P2Y12 signalling are particularly interesting if we consider the role these receptors play in microglial chemotaxis induced by adenine nucleotides [23] and the observation that microglia are the only cells belonging to the monocyte/macrophage lineage that express P2Y12 receptors [41]. If this holds true, P2Y12 receptors could be the only specific marker distinguishing microglia from peripheral macrophages so far identified.
Regarding P2Y14 receptors, we found mRNA expression by PCR but did not detect any Ca2+ response when using the endogenous agonist UDP-glucose. Nevertheless, since P2Y14 receptors are known to activate Gi/o proteins [42], we cannot exclude that the receptor proteins, if expressed, are able to accomplish other functions, such as the control of cyclic adenosine monophosphate (cAMP) accumulation. Interestingly, Bianco et al. [39] described the functional capability of P2Y14 receptors as highly dependent on cell activation. We can hypothesise that microglial activation promotes P2Y14 protein expression or coupling of the receptor to Ca2+ release. We should also consider that culture conditions slightly differing from lab to lab make it illusory to have a completely overlapping picture of a complex signalling system such as the P2Y, even more so in plastic cells such as microglia. Moreover, we found it interesting to consider some other aspects as causes of difference. Among these, the areas of the brain from which cell cultures come (cortex versus hippocampus), the developmental phase (embryonic versus post-natal) and species differences (rat versus murine N9 cell line). All of these aspects should be considered as interesting topics for further, more focused, investigations.
Ca2+ responses induced by adenine versus uracil nucleotides
According to our data, the Ca2+ signals triggered by adenine and uracil nucleotides differ from two main aspects: the main G protein involved and the more efficient induction of capacitative influx by uracil nucleotides. We utilised ADP to activate P2Y receptors sensitive to adenine nucleotides. According to inhibition by PTx, ADP-induced Ca2+ release occurred by a PTx-dependent mechanism while UTP-induced Ca2+ signals were almost unaffected by PTx pre-treatment. Involvement of different G proteins determined an early divergence of signal transduction pathways following receptor and G protein activation. In the case of uracil nucleotides, known to act mainly on Gq/11 and here found to be only partially PTx sensitive, Gα subunit would trigger PLCγ activation and Ca2+ release through IP3 receptor/channels [43]. In the case of P2Y12 receptors activated by ADP, the Gβγ subunit would trigger PLCβ activation and Ca2+ release while the Gαi subunit would probably cause a concomitant inhibition of adenylyl cyclase and cAMP reduction. However, even if the signalling pathways are so different, they converge to the same ER stores that the two nucleotides, when used at a concentration of 100 µM, deplete with similar efficacy, as depicted by cross-desensitisation experiments. This bring us to the other difference between the Ca2+ signalling induced by the two receptor types. Depletion of Ca2+ from the ER stores is known to be the starting signal of induction of the capacitative Ca2+ influx mainly described in non-excitable cells, among them microglia [21]. The nature and identity of the agent (or event) following Ca2+ depletion is still a matter of debate, and it might also be that different mechanisms put in place by ER Ca2+ depletion act in different cells [44]. In any case, in microglial cells, Ca2+ depletion might be necessary but certainly is not sufficient to cause capacitative Ca2+ influx. As already shown [21], uracil nucleotides (UTP and UDP) were clearly more efficient than ADP in inducing the Ca2+ plateau, which we identified as being due to capacitative influx on the basis of the sensitivity to SKF96395 and extracellular Ca2+.
Regardless the mechanisms utilised, the capability to trigger capacitative Ca2+ influx adds further functional capability to the receptors sensitive to uracil nucleotides, as depicted by the specificity of some functional outcomes linked to capacitative influx besides ER Ca2+ store replenishment: examples of this specificity are the modulation of some Ca2+-dependent adenylyl cyclase isoforms [45] and Ca2+-calmudulin-induced activation of nuclear factor of activated T cells (NFAT), which specifically need capacitative Ca2+ [44].
Oscillatory Ca2+ activity induced by low doses of nucleotides
The release of nucleotides can occur not only following cell lysis but also by highly regulated mechanisms in the course of cell activity, such as action potential propagation, synaptic activity and astrocytic Ca2+ waves [13, 27]. Can the different availability of nucleotides for receptor activation be utilised by microglia as an indicator of the condition of the host tissue? To address this question, we compared the Ca2+ signals triggered by P2Y receptors activated by long-lasting applications of low versus high doses of nucleotides. When ADP was applied at a low dose, only a single peak of Ca2+ was observed in a fraction of cells. On the contrary, when a low dose of UTP was used, we observed a more complex behaviour markedly differing from that induced by a high dose of the nucleotide: while this latter induced a single event of release followed by a long-lasting and slowly decaying phase of capacitative influx, a low dose of UTP (also of UDP: data not shown) caused an oscillatory Ca2+ activity only rarely superimposed to a slowly rising Ca2+ influx. Our data do not allow identification of a link between oscillatory release and Ca2+ influx, but we cannot exclude that oscillatory activity sustained for a time span longer than our experiments would need the contribution of Ca2+ influx. In recent years, a non-capacitative Ca2+ influx pathway elicited by low doses of metabotropic agonists, depending on arachidonic acid and responsible for the maintainence of Ca2+ oscillation, was described in a number of cells [46]. Moreover, calcineurin activation by capacitative Ca2+ entry was shown to inhibit the non-capacitative entry [34]. However, we did not detect any regulatory role played by a high dose of UTP or by the calcineurin inhibitor cyclosporin-A on the oscillatory Ca2+ activity.
An increasing body of evidence supports the relationship between cytoplasmic and mitochondrial Ca2+ changes [47, 48]. Energised mitochondria are known to take up Ca2+ accumulated in the cytoplasm, mainly thanks to the mitochondrial uniporter, and to release it back by the Na+/Ca2+ exchange system. Not only can mitochondria function as Ca2+ buffers, they also utilise the ion to regulate their own activity since the F1F0-ATP synthase and a number of mitochondrial dehydrogenases are regulated by Ca2+. It was suggested that mitochondria could increase the release of Ca2+ from IP3R channels by rapidly removing Ca2+ from their mouth, so decreasing Ca2+-induced inactivation of the channels. The Ca2+ taken by mitochondria could be recycled back to the Ca2+ ATPase on the ER to allow prompt refilling of the stores [49]. This would finally maintain the efficiency of the release process. When we utilised the protonophore FCCP, known to cause mitochondrial depolarisation, we could clearly see a decrease of oscillatory Ca2+ release induced by low doses of UTP. Interestingly, FCCP did not affect the potent release induced by a high dose of UTP (data not shown), as if for long-lasting oscillatory activity to occur in the presence of low IP3 synthesis a fine regulation of cytoplasmic Ca2+ was needed to maintain IP3R channels available to open. This is one of the first indications of the involvement of mitochondria in the control of cytoplasmic Ca2+ changes in microglia, and further investigation is needed to come to an unambiguous interpretation of this result since FCCP can have a number of outcomes together with the decrease of Ca2+ uptake by mitochondria, such as ATP depletion and pH changes. In any case, it is noteworthy that during microglia activation, mitochondria change their cellular distribution, morphology and internal organisation, probably to better sustain energy-consuming functions of activated microglia [50].
In this study, we pointed out the capability of P2Y receptors to cause different patterns of cytoplasmic Ca2+ changes involving capacitative Ca2+ influx and intracellular Ca2+ mobilisation, depending not only on the type but also on the concentration of the agonist. Intracellular Ca2+ changes can independently affect a multitude of functions and events depending on the vicinity of the Ca2+ sources to specific target proteins, on the concentration reached and also on the time evolution of the Ca2+ variations. It is known that sustained and oscillatory Ca2+ changes can differently affect target proteins (for a review, see 51). Protein kinases, phospholipases and transcription factors are examples of the multitude of proteins that can be differently regulated by Ca2+.
In conclusion we speculate that the different patterns of Ca2+ signals elicited by different concentrations of nucleotides would render P2Y receptors capable of signalling to microglia the state of the host tissue and, hopefully, regulate microglial responses in concert with the needs of the tissue.
References
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312′18
Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW (1999) Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev 30:77′05
Visentin S, Agresti C, Patrizio M, Levi G (1995) Ion channels in rat microglia and their different sensitivity to lipopolysaccharide and interferon-gamma. J Neurosci Res 42:439′51
De Simone R, Ajmone-Cat MA, Minghetti L (2004) Atypical antiinflammatory activation of microglia induced by apoptotic neurons: possible role of phosphatidylserine phosphatidylserine receptor interaction. Mol Neurobiol 29(2):197′12
Minghetti L (2005) Role of inflammation in neurodegenerative diseases. Curr Opin Neurol 18(3):315′21
Franke H, Illes P (2006) Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther 109(3):297′24
Dave S, Mogul DJ (1996) ATP receptor activation potentiates a voltage-dependent Ca channel in hippocampal neurons. Brain Res 715(1′):208′16
Ikeuchi Y, Nishizaki T (1995) Dual effects of ATP on the potassium channel and intracellular Ca2+ release in smooth muscle cells of the bovine brain arteries. Biochem Biophys Res Commun 215(3):1071′077
Wirkner K, Koles L, Thummler S, Luthardt J, Poelchen W, Franke H, Furst S, Illes P (2002) Interaction between P2Y and NMDA receptors in layer V pyramidal neurons of the rat prefrontal cortex. Neuropharmacology 42(4):476′88
Abbracchio MP, Saffrey MJ, Hopker V, Burnstock G (1994) Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience 59(1):67′6
Ciccarelli R, Di Iorio P, Ballerini P, Ambrosini G, Giuliani P, Tiboni GM, Caciagli F (1994) Effects of exogenous ATP and related analogues on the proliferation rate of dissociated primary cultures of rat astrocytes. J Neurosci Res 39(5):556′66
Franke H, Krugel U, Illes P (1999) P2 receptor-mediated proliferative effects on astrocytes in vivo.Glia 28(3):190′00
Venance L, Stella N, Glowinski J, Giaume C (1997) Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. J Neurosci 17(6):1981′992
Fields RD, Stevens B (2000) ATP: an extracellular signalling molecule between neurons and glia. Trends Neurosci 23:625′33
Agresti C, Meomartini ME, Amadio S, Ambrosini E, Volonte C, Aloisi F, Visentin S (2005) ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res Brain Res Rev 48(2):157′65
Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F (1997) Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med 185(3):579′82
Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y (2000) Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem 75(3):965′72
Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Collo G, Buell G, Di Virgilio F (1997) ATP-mediated cytotoxicity in microglial cells. Neuropharmacology 36(9):1295′301
Visentin S, Renzi M, Frank C, Greco A, Levi G (1999) Two different ionotropic receptors are activated by ATP in rat microglia. J Physiol 519(Pt 3):723′36
Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424(6950):778′83
Toescu EC, Moller T, Kettenmann H, Verkhratsky A (1998) Long-term activation of capacitative Ca2+ entry in mouse microglial cells. Neuroscience 86(3):925′35
Moller T, Kann O, Verkhratsky A, Kettenmann H (2000) Activation of mouse microglial cells affects P2 receptor signaling. Brain Res 853(1):49′9
Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S (2001) Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci 21(6):1975′982
Ogata T, Chuai M, Morino T, Yamamoto H, Nakamura Y, Schubert P (2003) Adenosine triphosphate inhibits cytokine release from lipopolysaccharide-activated microglia via P2y receptors. Brain Res 981(1′):174′83
Seo DR, Kim KY, Lee YB (2004) Interleukin-10 expression in lipopolysaccharide-activated microglia is mediated by extracellular ATP in an autocrine fashion. Neuroreport 15(7):1157′161
Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K (2001) Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem 78(6):1339′349
Verderio C, Matteoli M (2001) ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. J Immunol 166(10):6383′391
Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362(4′):299′09
Braun N, Zhu Y, Krieglstein J, Culmsee C, Zimmermann H (1998) Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J Neurosci 18(13):4891′900
Gallucci S, Matzinger P (2001) Danger signals: SOS to the immune system. Curr Opin Immunol 13(1):114′19
la Sala A, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G (2003) Alerting and tuning the immune response by extracellular nucleotides. J Leukoc Biol 73(3):339′43
Levi G, Patrizio M, Bernardo A, Petrucci TC, Agresti C (1993) Human immunodeficiency virus coat protein gp120 inhibits the beta-adrenergic regulation of astroglial and microglial functions. Proc Natl Acad Sci USA 90(4):1541′545
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260(6):3440′450
Mignen O, Thompson JL, Shuttleworth TJ (2003) Calcineurin directs the reciprocal regulation of calcium entry pathways in nonexcitable cells. J Biol Chem 278(41):40088′0096
Hoth M, Fanger CM, Lewis RS (1997) Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol 137(3):633′48
Kettenmann H, Banati R, Walz W (1993) Electrophysiological behavior of microglia. Glia 7(1):93′01
Norenberg W, Langosch JM, Gebicke-Haerter PJ, Illes P (1994) Characterization and possible function of adenosine 5′triphosphate receptors in activated rat microglia. Br J Pharmacol 111(3):942′50
Langosch JM, Gebicke-Haerter PJ, Norenberg W, Illes P (1994) Characterization and transduction mechanisms of purinoceptors in activated rat microglia. Br J Pharmacol 113(1):29′4
Bianco F, Fumagalli M, Pravettoni E, D’Ambrosi N, Volonte C, Matteoli M, Abbracchio MP, Verderio C (2005) Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev 48(2):144′56
Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409(6817):202′07
Sasaki Y, Hoshi M, Akazawa C, Nakamura Y, Tsuzuki H, Inoue K, Kohsaka S (2003) Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia 44(3):242′50
Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G (2003) Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci 24(2):52′5
Communi D, Janssens R, Suarez-Huerta N, Robaye B, Boeynaems JM (2000) Advances in signalling by extracellular nucleotides: the role and transduction mechanisms of P2Y receptors. Cell Signal 12(6):351′60
Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL (2002) The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol 4(11):E263–E272
Fagan KA, Mons N, Cooper DM (1998) Dependence of the Ca2+-inhibitable adenylyl cyclase of C6-2B glioma cells on capacitative Ca2+ entry. J Biol Chem 273(15):9297′305
Shuttleworth TJ, Mignen O (2003) Calcium entry and the control of calcium oscillations. Biochem Soc Trans 31(Pt 5):916′19
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287(4):C817–C833
Duchen MR (2004) Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med 25(4):365′51
Poburko D, Kuo KH, Dai J, Lee CH, van Breemen C (2004) Organellar junctions promote targeted Ca2+ signaling in smooth muscle: why two membranes are better than one. Trends Pharmacol Sci 25(1):8′5
Banati RB, Egensperger R, Maassen A, Hager G, Kreutzberg GW, Graeber MB (2004) Mitochondria in activated microglia in vitro. J Neurocytol 33(5):535′41
Johnson JD, Chang JP (2000) Function- and agonist-specific Ca2+ signalling: the requirement for and mechanism of spatial and temporal complexity in Ca2+ signals. Biochem Cell Biol 78(3):217′40
Acknowledgement
The authors thank Mrs. Estella Sansonetti for excellent technical and editorial assistance. This work was supported by the Italian Ministry of Education, University and Research (FIRBMIUR, Grant no. H91/1: “Synaptic plasticity and brain repair.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Visentin, S., Nuccio, C.D. & Bellenchi, G.C. Different patterns of Ca2+ signals are induced by low compared to high concentrations of P2Y agonists in microglia. Purinergic Signalling 2, 605–617 (2006). https://doi.org/10.1007/s11302-006-9023-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-006-9023-1