Abstract
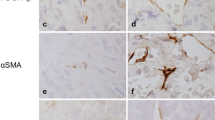
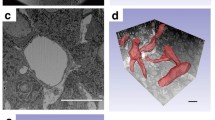
Pericytes are perivascular cells associated with microcirculation. Typically, they are localized close to the capillary wall, underneath the basement membrane, and have sparse cytoplasm and poorly developed cell organelles. However, the specific properties of pericytes vary by organ and the conditions within organs. We recently demonstrated that pericytes in rat anterior pituitary gland produce type I and III collagens. The present study attempted to determine the morphological characteristics of these pituitary pericytes. Castrated rats were used as a model of hormonal and vascular changes in the gland. Pericytes, as determined by desmin immunohistochemistry, were more numerous and stained more intensely in castrated rats. Transmission electron microscopy revealed that pituitary pericytes displayed the typical characteristics of pericytes. In pituitary sections from castrated rats, the Golgi apparatus of pericytes was well developed and the rough endoplasmic reticulum was elongated. Additionally, scanning electron microscopy revealed four pericyte shapes: oval, elongate, triangular, and multiangular. As compared with normal rats, the proportion of oval pericytes was lower, and the proportions of the other three shapes were higher, in castrated rats. These results suggest that pericytes change their fine structure and cell shape in response to hormonal and vascular changes in the anterior pituitary gland. In addition, a novel type of perivascular cell was found by desmin immunoelectron microscopy. The morphological properties of these cells were dissimilar to those of pericytes. The cells were localized in the perivascular space, had no basement membrane, and contained dilated rough endoplasmic reticulum. This new cell type will require further study of its origin and characteristics.








Similar content being viewed by others
References
Alba C, Vidal L, Díaz F, Villena A, de Vargas IP (2004) Ultrastructural and quantitative age-related changes in capillaries of the dorsal lateral geniculate nucleus. Brain Res Bull 64:145–153
Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 7:452–464
Chaturvedi K, Sarkar DK (2006) Isolation and characterization of rat pituitary endothelial cells. Neuroendocrinology 83:387–393
Díaz-Flores L, Gutiérrez R, Varela H, Rancel N, Valladares F (1991) Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol 6:269–286
Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA (2000) Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res 60:55–69
Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK (2006) Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev 20:486–500
Fujiwara K, Jindatip D, Kikuchi M, Yashiro T (2010) In situ hybridization reveals that type I and III collagens are produced by pericytes in the anterior pituitary gland of rats. Cell Tissue Res 342:491–495
Gay VL, Midgley AR Jr (1969) Response of the adult rat to orehidectomy and ovariectomy as determined by LH radioimmunoassay. Endocrinology 84:1359–1364
Hashizume H, Ushiki T (2002) Three-dimensional cytoarchitecture of angiogenic blood vessels in a gelatin sheet implanted in the rat skeletal muscular layers. Arch Histol Cytol 65:347–357
Higuchi K, Hashizume H, Aizawa Y, Ushiki T (2000) Scanning electron microscopic studies of the vascular smooth muscle cells and pericytes in the rat heart. Arch Histol Cytol 63:115–126
Hirschi KK, D’Amore PA (1996) Pericytes in the microvasculature. Cardiovasc Res 32:687–698
Hughes S, Chan-Ling T (2004) Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci 45:2795–2806
Inoue K, Tanaka S, Kurosumi K (1985) Mitotic activity of gonadotropes in the anterior pituitary of the castrated male rat. Cell Tissue Res 240:271–276
Inoue K, Couch EF, Takano K, Ogawa S (1999) The structure and function of folliculo-stellate cells in the anterior pituitary gland. Arch Histol Cytol 62:205–218
Itoh J, Serizawa A, Kawai K, Ishii Y, Teramoto A, Osamura RY (2003) Vascular networks and endothelial cells in the rat experimental pituitary glands and in the human pituitary adenomas. Microsc Res Tech 60:231–235
Kamba T, Tam BY, Hashizume H et al (2005) VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290:H560–H576
Mandarino LJ, Sundarraj N, Finlayson J, Hassell HR (1993) Regulation of fibronectin and laminin synthesis by retinal capillary endothelial cells and pericytes in vitro. Exp Eye Res 57:609–621
Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM (2002) Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 160:985–1000
Nihei A, Hagiwara K, Kikuchi M, Yashiro T, Hoshino Y (2003) Histological investigation of rabbit ligamentum flavum with special reference to differences in spinal levels. Anat Sci Int 78:162–167
Pfister F, Feng Y, vom Hagen F et al (2008) Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 57:2495–2502
Reddy K, Zhou Z, Jia SF, Lee TH, Morales-Arias J, Cao Y, Kleinerman ES (2008) Stromal cell-derived factor-1 stimulates vasculogenesis and enhances Ewing’s sarcoma tumor growth in the absence of vascular endothelial growth factor. Int J Cancer 123:831–837
Sarkar AJ, Chaturvedi K, Chen CP, Sarkar DK (2007) Changes in thrombospondin-1 levels in the endothelial cells of the anterior pituitary during estrogen-induced prolactin-secreting pituitary tumors. Endocrinology 192:395–403
Schaeffer M, Hodson DJ, Lafont C, Mollard P (2010) Functional importance of blood flow dynamics and partial oxygen pressure in the anterior pituitary. Eur J Neurosci 32:2087–2095
Shepro D, Morel NM (1993) Pericyte physiology. FASEB J 7:1031–1038
Shi X (2009) Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Am J Pathol 174:1692–1704
Tilton RG (1991) Capillary pericytes: perspectives and future trends. J Electron Microsc Tech 19:327–344
Tozer GM, Akerman S, Cross NA et al (2008) Blood vessel maturation and response to vascular-disrupting therapy in single vascular endothelial growth factor-A isoform-producing tumors. Cancer Res 68:2301–2311
Wallow IH, Bindley CD, Linton KL, Rastegar D (1991) Pericyte changes in branch retinal vein occlusion. Invest Ophthalmol Vis Sci 32:1455–1463
Acknowledgments
We are grateful to Prof. Tatsuo Ushiki and Dr. Daisuke Koga (Niigata University, Japan) for technical advice on preparing tissues for scanning electron microscopy. We thank Megumi Yatabe (Jichi Medical University, Japan) for her suggestions on transmission electron microscopy procedure. We also thank David Kipler of Supernatant Communications for revising the language of the manuscript. This work was supported in part by promotional funds for the Keirin Race of the Japan Keirin Association, the Research Award to JMU Graduate student, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to T.Y. (22590192).
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
12565_2012_144_MOESM1_ESM.ppt
Supplementary Fig. 1 Double immunofluorescent histochemistry of polyclonal anti-desmin antibody (a, c, red) and monoclonal anti-desmin antibody (b, c, green). The section was incubated with anti-human desmin mouse monoclonal antibody (diluted 1:100, clone D33; Dako, Glostrup, Denmark) and then treated with Alexa Fluor 488-conjugated goat anti-mouse IgG (dilated 1:200; Invitrogen, Carlsbad, CA). After the process of immunostaining against the monoclonal antibody was complete, another immunostaining was performed using a polyclonal primary antibody and Alexa Fluor goat 568-conjugated anti-rabbit IgG (Invitrogen). Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) (blue). Fluorescent images were visualized with a confocal laser microscope (FV1000; Olympus, Tokyo, Japan). All polyclonal anti-desmin-immunopositive cells also show monoclonal anti-desmin immunoreactivity (c). Bars 10 μm (PPT 602 kb)
Rights and permissions
About this article
Cite this article
Jindatip, D., Fujiwara, K., Kouki, T. et al. Transmission and scanning electron microscopy study of the characteristics and morphology of pericytes and novel desmin-immunopositive perivascular cells before and after castration in rat anterior pituitary gland. Anat Sci Int 87, 165–173 (2012). https://doi.org/10.1007/s12565-012-0144-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-012-0144-z