Abstract
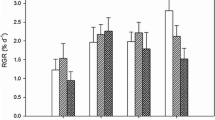
Deforestation of the kelp Ecklonia cava has been widely reported in Japan, and may be due to physiological stress caused by warm seawater temperatures and low nitrogen availability. To test this hypothesis, we examined the effects of temperature (20, 26, 28, and 30 °C) and nitrogen conditions (seawater enriched with 25 % PESI vs. non-enriched seawater) on photosynthesis, growth and survival, and nitrogen and chlorophyll a content of juvenile sporophytes (3–4 cm) along the coast of the Izu Peninsula, Japan. The juvenile sporophytes cultured in enriched medium showed significantly greater photosynthetic activity and relative growth rates than those cultured in non-enriched seawater at 20–28 °C, likely because of the much higher nitrogen and chlorophyll a content in the enriched medium. A significant effect of temperature on photosynthesis and relative growth rates was also detected under both nitrogen conditions. However, in contrast to the excellent survival exhibited in the enriched medium, dead juveniles were observed in non-enriched seawater at all temperatures, and survival rates decreased with increasing temperature. These results reveal that growth and survival of E. cava juvenile sporophytes are negatively affected by low nitrogen availability and high seawater temperature, which may result in deforestation of this kelp.




Similar content being viewed by others
References
Schiel DR, Foster MS (1986) The structure of subtidal algal stands in temperate waters. Oceanogr Mar Biol Annu Rev 24:265–307
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Smale DA, Burrows MT, Moore PJ, Connor N, Hawkins SJ (2013) Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol Evol 3:4016–4038
Kirihara S, Nakamura T, Kon N, Fujita D, Notoya M (2006) Recent fluctuations in distribution and biomass of cold and warm temperature species of Laminarialean algae at Cape Ohma, northern Honshu, Japan. J Appl Phycol 18:521–527
Suzuki S, Furuya K, Kawai T, Takeuchi I (2008) Effect of seawater temperature on the productivity of Laminaria japonica in the Uwa Sea, southern Japan. J Appl Phycol 20:833–844
Johnson CR, Banks SC, Barrett NS, Cazassus F, Dunstan PK, Edgar GJ, Frusher SD, Gardner C, Haddon M, Helidoniotis F, Hill KL, Holbrook NJ, Hosie GW, Last PR, Ling SD, Thomas JM, Miller K, Pecl GT, RichardsonI AJ, Ridgway KR, Rintoul SR, Ritz DA, Ross DJ, Sanderson JC, Shepherd SA, Slotwinski A, Swadling KM, Taw N (2011) Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. J Exp Mar Biol Ecol 400:17–32
Wernberg T, Russell BD, Moore PJ, Ling SD, Smale DA, Campbell A, Coleman MA, Steinberg PD, Kendrick GA, Connell SD (2011) Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. J Exp Mar Biol Ecol 400:7–16
Díez I, Muguerza N, Santolaria A, Ganzedo U, Gorostiaga JM (2012) Seaweed assemblage changes in the eastern Cantabrian Sea and their potential relationship to climate change. Estuar Coast Shelf Sci 99:108–120
Moy FE, Christie H (2012) Large-scale shift from sugar kelp (Saccharina latissima) to ephemeral algae along the south and west coast of Norway. Mar Biol Res 8:309–321
Jackson GA (1977) Nutrients and production of the giant kelp Macrocystis pyrifera off southern California. Limnol Oceanogr 22:979–995
Zimmerman RC, Kremer JN (1984) Episodic nutrient supply to a kelp forest ecosystem in Southern California. J Mar Res 42:591–604
North WJ, Zimmerman RC (1984) Influences of macronutrients and water temperature on summertime survival of Macrocystis canopies. Hydrobiologia 116(117):419–424
Dean TA, Jacobsen FR (1986) Nutrient-limited growth of juvenile kelp, Macrocystis pyrifera, during the 1982–1984 “El Niño” in southern California. Mar Biol 90:597–601
Tegner MJ, Dayton PK, Edwards PB, Riser KL (1997) Large-scale, low-frequency oceanographic effects on kelp forest succession: a tale of two cohorts. Mar Ecol Prog Ser 146:117–134
Dayton PK, Tegner MJ, Parnell PE, Edward PB (1999) Temporal and spatial scales of kelp demography: the role of oceanographic climate. Ecol Monogr 69:219–250
Hernández-Carmona G, Robledo D, Serviere-Zaragoza E (2001) Effect of nutrient availability on Macrocystis pyrifera recruitment and survival near its southern limit off Baja California. Bot Mar 44:221–229
Bolton JJ, Lünning K (1982) Optimal growth and maximal survival temperatures of Atlantic Laminaria species (Phaeophyta) in culture. Mar Biol 66:89–94
Morita T, Kurashima A, Maegawa M (2003) Temperature requirements for the growth and maturation of the gametophytes of Undaria pinnatifida and U. undarioides (Laminariales, Phaeophyta). Phycol Res 51:154–160
Komazawa I, Sakanishi Y, Tanaka J (2015) Temperature requirements for growth and maturation of the warm temperature kelp Eckloniopsis radicosa (Laminariales, Phaeophyta). Phycol Res 63:64–71
Hoffmann AJ, Avila M, Santelices B (1984) Interactions of nitrate and phosphate on the development of microscopic stages of Lessonia nigrescens Bory (Phaeophyta). J Exp Mar Biol Ecol 78:177–186
Mizuta H, Narumi H, Yamamoto H (2001) Effects of nitrate and phosphate on the growth and maturation of gametophytes of Laminaria religiosa Miyabe (Phaeophyceae). Suisanzoshoku 49:175–180
Carney LT, Edwards MS (2010) Role of nutrient fluctuations and delayed development in gametophyte reproduction by Macrocystis pyrifera (Phaeophyceae) in southern California. J Phycol 46:987–996
Dean TA, Jacobsen FR (1984) Growth of juvenile Macrocystis pyrifera (Laminariales) in relation to environmental factors. Mar Biol 83:301–311
Kawashima S (1993) An illustrated book of Japanese Laminariales. Kita Nihon Kaiyo Center, Sapporo (in Japanese)
Iwahashi Y, Inaba S, Fushimi H, Sasaki T, Osuga H (1979) Ecological studies on Eisenia and Ecklonia in the coast of Izu Peninsula-IV. The distribution and characteristics of kelp stand. Bull Shizuoka Prefect Fish Exp Stn 13:75–82 (in Japanese)
Ohno M (1985) Marine forest–its ecology and constructing technology. Kaiyoukagaku 17:706–713 (in Japanese)
Yokohama Y, Tanaka J, Chihara M (1987) Productivity of the Ecklonia cava community in a bay of Izu Peninsula on the Pacific coast of Japan. Bot Mag Tokyo 100:129–141
Tominaga H, Serisawa Y, Ohno M (2004) Seasonal changes in net production of the bladelets and size of the proximal blade of Ecklonia cava in Tosa Bay, Kochi Prefecture. Jpn J Phycol 52:13–19 (in Japanese)
Arakawa H, Ido M, Arimoto M, Agatsuma Y (2013) Combined effects of high water temperature and low flow velocity on survival of brown algae Eisenia bicyclis and Ecklonia cava. Can J Plant Prot 1(4):125–128
Nakayama Y, Arai S (1999) Grazing of the brown alga Ecklonia cava by three herbivorous fishes on the coast of Nakagi, South Izu, central Japan. Jpn J Phycol 47:105–112 (in Japanese)
Tatewaki M (1966) Formation of a crustose sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia 6:62–66
Yokohama Y, Tanaka J, Chihara M (1987) Productivity of the Ecklonia cava community in a Bay of Izu Peninsula on the Pacific coast of Japan. Bot Mag Tokyo 100:129–141
Bird NL, Chen LCM, Mclachlan J (1979) Effects of temperature, light and salinity on growth in culture of Chondrus crispus, Furcellaria lumbricalis, Gracilaria tikvahiae (Gigartinales, Rhodophyta) and Fucus serratus (Fucales, Phaeophyta). Bot Mar 22:521–527
Ohno M, Largo DB, Ikumoto T (1994) Growth rate, carrageenan yield and gel properties of cultured kappa-carrageenan producing red alga Kappaphycus alvarezii (Doty) Doty in the subtropical waters of Shikoku, Japan. J Appl Phycol 6:1–5
Yokohama Y, Katayama N, Furuya K (1986) An improved type of “Product-meter”, a differential gas-volumeter, and its application to measuring photosynthesis of seaweeds. Jpn J Phycol 34:37–42 (in Japanese)
Duarte P, Ferreira JG (1995) Seasonal adaptation and short-term metabolic responses of Gelidium sesquipedale to varying light and temperature. Mar Ecol Prog Ser 121:289–300
Inskeep WP, Bloom PR (1985) Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80 % acetone. Plant Physiol 77:483–485
Wheeler PA, North WJ (1980) Effect of nitrogen supply on nitrogen content and growth rates of juvenile Macrocystis pyrifera sporophytes. J Phycol 16:577–582
Deysher LE, Dean TA (1986) In situ recruitment of sporophytes of giant kelp Macrocystis pyrifera (L.) Agardh CA: effects of physical factors. J Exp Mar Biol Ecol 103:41–63
Gerard VA (1997) The role of nitrogen nutrition in high-temperature tolerance of the kelp Laminaria saccharina (Chromophyta). J Phycol 33:800–810
Chapman ARO, Markham JW, Lünning K (1978) Effects of nitrate concentration on the growth and physiology of Laminaria saccharina (Phaeophyta) in culture. J Phycol 14:195–198
Lapointe BE (1981) The effects of light and nitrogen on growth, pigment content and biochemical composition of Gracilaria foliifera V. Angustissima (Gigartinales, Rhodophyta). J Phycol 17:90–95
Bird KT, Habig C, Debusk T (1982) Nitrogen allocation and storage patterns in Gracilaria tikvahiae (Rhodophyta). J Phycol 18:344–348
Lingnell A, Pedersen M (1987) Nitrogen metabolism in Gracilaria secundata (Harv.) Hydrobiologia151/152:431–441
Levy I, Gantt E (1990) Development of photosynthetic activity in Porphyridium purpureum (Rhodophyta) following nitrogen starvation. J Phycol 26:62–68
Dean PR, Hurd CL (2007) Seasonal growth, erosion rates, and nitrogen and photosynthetic ecophysiology of Undaria pinnatifida (Heterokontophyta) in southern New Zealand. J Phycol 43:1138–1148
Gagné JA, Mann KH, Chapman ARO (1982) Seasonal patterns of growth and storage in Laminaria longicruris in relation to differing patterns of availability of nitrogen in the water. Mar Biol 69:91–101
Serisawa Y, Yokohama Y, Aruga Y, Bellgrove A (2004) Photosynthetic performance of transplanted ecotypes of Ecklonia cava (Laminariales, Phaeophyta). J Appl Phycol 16:227–235
Agatsuma Y, Endo H, Yoshida S, Ikemori C, Takeuchi Y, Fujishima H, Nakajima K, Sano M, Kanezaki N, Imai H, Yamamoto N, Kanahama H, Matsubara T, Takahashi S, Isogai T, Taniguchi K (2014) Enhancement of Saccharina kelp production by nutrient supply in the Sea of Japan off southwestern Hokkaido, Japan. J Appl Phycol 26:1845–1852
Acknowledgments
We sincerely thank Professor Emeritus K. Taniguchi (deceased) of Tohoku University for supporting this study. We also thank Professor O. Nishimura and Dr. K. Ito of Tohoku University for helping with the analysis of seawater nutrient concentrations and nitrogen and chlorophyll a content. This study was supported in part by a grant-in-aid from the Japanese Society for Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, X., Endo, H., Nagaki, M. et al. Growth and survival of juvenile sporophytes of the kelp Ecklonia cava in response to different nitrogen and temperature regimes. Fish Sci 82, 623–629 (2016). https://doi.org/10.1007/s12562-016-0998-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-016-0998-4