Abstract
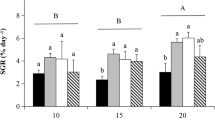
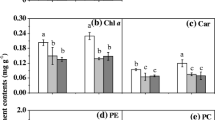
Young sporophytes of Sargassum fusiforme were cultured at decreased CO2 (20 μatm), ambient CO2 (400 μatm), and high CO2 (1000 μatm), and then the quantum efficiency of open photosystem II (Fv′/Fm′), initial slope of the rapid light curves (α), and relative maximum photosynthetic electron transport rate (rETRm) of the algae under different temperatures and light levels were measured. The study aimed to investigate how the decreased CO2 and high CO2 supply affected the growth and photosynthetic functions of S. fusiforme young sporophytes. While both lowered and increased CO2 supply significantly reduced the growth rates of the alga, greater declines were observed under decreased CO2. The Fv′/Fm′, α, and rETRm of alga remained stable after short-term (120 min) exposures to 18, 22, and 26 °C, as well as to highlight (300 μmol photons m−2 s−1), with no significant difference among the three CO2 supply treatments. Hence, neither decreased nor increased CO2 affected the photosynthetic responses of S. fusiforme young sporophytes to temperature and high light. However, the Fv′/Fm′ of the three CO2 treatments declined by 72% under 60 μmol photons m−2 s−1, suggesting its sensitivity to short-term low light. These observations are crucial for the improved management of S. fusiforme for commercial farming, while ensuring its sustainable production and supply amid seawater pH shifts brought about by global climate change.








Similar content being viewed by others
References
Andría JR, Brun FG, Pérez-Lloréns JL, Vergara JJ (2001) Acclimation responses of Gracilaria sp. (Rhodophyta) and Enteromorpha intestinalis (Chlorophyta) to changes in the external inorganic carbon concentration. Bot Mar 44:361–370
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170:489–504
Caldeira K, Wickett ME (2003) Oceanography: anthropogenic carbon and ocean pH. Nature 425:365
Chung IK, Oak JH, Lee JA, Shin JA, Kim GJ, Park KS (2013) Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean project overview. ICES J Mar Sci 11:1–7
Davison IR (1991) Environmental effects on algal photosynthesis: temperature. J Phycol 27:2–8
Edwards KF, Thomas MK, Klausmeier CA, Litchman E (2016) Phytoplankton growth and the interaction of light and temperature: a synthesis at the species and community level. Limnol Oceanogr 61:1232–1244
Friedlander M, Levy I (1995) Cultivation of Gracilaria in outdoor tanks and ponds. J Appl Phycol 7:315–324
Gao KS, Aruga YS, Asada K, Ishihara T, Akano T, Kiyohara M (1991) Enhanced growth of the red alga Porphyra yezoensis Ueda in high CO2 concentrations. J Appl Phycol 3:356–362
García-Sânchez MJ, Fernândez JA, Niell FX (1994) Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta 194:55–61
Gordillo FJL, Niell FX, Figueroa FLL (2001) Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 213:64–70
Gordillo FJL, Figueroa FL, Niell FX (2003) Photon- and carbon-use efficiency in Ulva rigida at different CO2 and N levels. Planta 218:315–322
Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA, Graham MH (2012) Effects of climate change on global seaweed communities. J Phycol 48:1064–1078
Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X et al (2001) Climate change 2001: the scientific basis. Cambridge: Cambridge University Press. 881 pp
Israel A, Friedlander M (1998) Inorganic carbon utilisation and growth abilities in the marine red macroalga Gelidiopsis sp. Isr J Plant Sci 46:117–124
Israel A, Katz S, Dubinsky Z, Merrill JE, Friedlander M (1999) Photosynthetic inorganic carbon utilization and growth of Porphyra linearis (Rhodophyta). J Appl Phycol 11:447–453
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Jiang H, Zou DH, Li XH (2016a) Growth, photosynthesis and nutrient uptake by Grateloupia livida (Halymeniales, Rhodophyta) in response to different carbon levels. Phycologia 55:462–468
Jiang H, Zou DH, Chen BB (2016b) Effects of lowered carbon supplies on two farmed red seaweeds, Pyropia haitanensis (Bangiales) and Gracilaria lemaneiformis (Gracilariales), grown under different sunlight conditions. J Appl Phycol 28:3469–3477
Jiang H, Zou DH, Chen WZ, Yang YF (2017a) The photosynthetic responses to stocking depth and algal mat density in the farmed seaweed Gracilaria lemaneiformis (Gracilariales, Rhodophyta). Environ Sci Pollut Res 24:25309–25314
Jiang H, Zou DH, Lou WY, Ye CP (2017b) Effects of stocking density and decreased carbon supply on the growth and photosynthesis in the farmed seaweed, Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol 29:3057–3065
Jiang H, Zou DH, Lou WY, Deng YY, Zeng XP (2018) Effects of seawater acidification and alkalization on the farmed seaweed, Pyropia haitanensis (Bangiales, Rhodophyta), grown under different irradiance conditions. Algal Res 31:413–420
Koch M, Bowes G, Ross C, Zhang XH (2013) Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob Chang Biol 19:103–132
Kübler JE, Davison IR (1995) Thermal acclimation of light use characteristics of Chondrus crispus (Rhodophyta). Eur J Phycol 30:189–195
Kurkdjian A, Guern J (1989) Intracellular pH: measurement and importance in cell activity. Annu Rev Plant Physiol Plant Mol Biol 40(1):271–303
Larcher S (1994) Photosynthesis as a tool for indicating temperature stress events. In: Schulze ED, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, pp 261–277
Larcher S (2000) Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosystems 134:279–295
Liu YT, Xu JT, Gao KS (2017) CO2-driven seawater acidification increases photochemical stress in a green alga. Phycologia 51:562–566
Lopes PF, Oliveira MC, Colepicolo P (1997) Diurnal fluctuation of nitrate reductase activity in the marine red alga Gracilaria tenuistpitata (Rhodophyta). J Phycol 33:225–231
Lu CM, Chau CW, Zhang JH (2000) Acute toxicity of excess mercury on the photosynthetic performance of cyanobacterium, S. platensis-assessment by chlorophyll fluorescence analysis. Chemosphere 41:191–196
Machalek KM, Davison IR, Falkowski PG (1996) Thermal acclimation and photoacclimation of photosynthesis in the brown alga Laminaria saccharina. Plant Cell Environ 19:1005–1016
Mercado JM, Javier F, Gordillo L, Niell FX, Figueroa FL (1999) Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J Appl Phycol 11:455–461
Millero FJ (2007) The marine inorganic carbon cycle. Chem Rev 107:308–341
Pang SJ, Zhang ZH, Zhao HJ, Sun JZ (2007) Cultivation of the brown alga Hizikia fusiformis (Harvey) Okamura: stress resistance of artificially raised young seedlings revealed by chlorophyll fluorescence measurement. J Appl Phycol 19:557–565
Ralph PJ (1998) Photosynthetic response of laboratory-cultured Halophila ovalis to thermal stress. Mari Ecol Prog Ser 171:123–130
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Ralph PJ, Gademann R, Larkum AWD, Schreiber U (1999) In situ underwater measurements of photosynthetic activity of coral zooxanthellae and other reef-dwelling dinoflagellate endosymbionts. Mar Ecol Prog Ser 180:139–147
Richards DK, Hurd CL, Pritchard DW, Wing SR, Hepburn CD (2011) Photosynthetic response of monospecific macroalgal stands to density. Aquat Biol 13:41–49
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, pp 49–70
Smith FA, Raven JA (1979) Intracellular pH and its regulation. Annu Rev Plant Biol 30:289–311
Solomon S, Qin D, Manning M, Marquis M, Averyt K, Tignor MMB, Miller Jr HL, Chen Z, Eds (2007) Climate change 2007: the physical science basis. Cambridge University Press, 996 pp.
Stumm W, Morgan JJ (1981) Aquatic Chemistry, Wiley, New York. 583 p
Suárez-Álvarez S, Gómez-Pinchetti JL, García-Reina G (2012) Effects of increased CO2 levels on growth, photosynthesis, ammonium uptake and cell composition in the macroalga Hypnea spinella (Gigartinales, Rhodophyta). J Appl Phycol 24:815–823
Takahashi T, Feely RA, Weiss RF, Wanninkhof RH, Chipman DW, Sutherland SC, Takahashi TT (1997) Global air-sea flux of CO2: an estimate based on measurements of sea–air pCO2 difference. Proc Nat Acad Sci USA 94:8292–8299
Talarico L, Maranzana G (2000) Light and adaptive responses in red macroalgae: an overview. J Photochem Photobiol B 56:1–11
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Xu JT, Gao KS (2012) Future CO2-induced ocean acidification mediates the physiological performance of a green tide alga. Plant Physiol 160:1762–1769
Zacher K, Roleda MY, Hanelt D, Wiencke C (2007) UV effects on photosynthesis and DNA in propagules of three Antarctic seaweeds (Adenocystis utricularis, Monostroma hariotii and Porphyra endiviifolium). Planta 225:1505–1516
Zou DH (2014) The effects of severe carbon limitation on the green seaweed, Ulva conglobata (Chlorophyta). J Appl Phycol 26:2417–2424
Zou DH, Gao KS (2009) Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) grown at different irradiance levels. Phycologia 48:510–517
Zou DH, Gao KS (2010a) Physiological responses of seaweeds to elevated atmospheric CO2 concentrations. In: Israel A, Einav R, Seckbach J (eds) Seaweeds and their role in globally changing environment. Springer, Berlin, pp 115–126
Zou D, Gao K (2010b) Photosynthetic acclimation to different light levels in the brown marine macroalga, Hizikia fusiformis (Sargassaceae, Phaeophyta). J Appl Phycol 22:395–404
Zou DH, Gao KS, Xia JR (2003) Photosynthetic utilization of inorganic carbon in the economic brown alga, Hizikia fusiforme (Sargassaceae) from the South China Sea. J Phycol 39:1095–1100
Zou DH, Gao KS, Ruan ZX (2006) Seasonal pattern of reproduction of Hizikia fusiformis (Sargassaceae, Phaeophyta) from Nanao Island, Shantou, China. J Appl Phycol 18:195–201
Zou DH, Gao KS, Chen WZ (2011) Photosynthetic carbon acquisition in Sargassum henslowianum (Fucales, Phaeophyta), with special reference to the comparison between the vegetative and reproductive tissues. Photosynth Res 107:159–168
Zou D, Gao K, Harley C (2014) Temperature response of photosynthetic light- and carbon-use characteristics in the red seaweed Gracilaria lemaneiformis (Gracilariales, Rhodophyta). J Phycol 50:366–375
Acknowledgements
This study was supported by the Science and Technology Planning Project of Guangdong Province (2016A020222001), National Natural Science Foundation of China (No. 31741018), and project funded by China Postdoctoral Science Foundation (2018M633044).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, H., Zou, D., Lou, W. et al. Effects of CO2 supply on growth and photosynthetic ability of young sporophytes of the economic seaweed Sargassum fusiforme (Sargassaceae, Phaeophyta). J Appl Phycol 31, 615–624 (2019). https://doi.org/10.1007/s10811-018-1569-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1569-0