Abstract
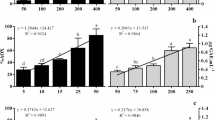
This study was performed to compare the various bioactivities of water-soluble, polar, and nonpolar extracts from the edible part (gonad) and waste (body wall) of the sea urchin Tripneustes gratilla. During antioxidant activity screening, these three extracts from both gonad and body wall showed various antioxidant activities in different tests. During human skin fibroblast (CCD966SK) viability and collagen screening, only water-soluble and polar extracts from gonad and body wall were observed to promote cell viability, while the water-soluble gonad extract promoted the collagen-generating activity of CCD966SK cells. The water-soluble and polar extracts from gonad and body wall showed proliferative activity towards different cell types. In contrast, the nonpolar extracts of gonad and body wall exerted antiproliferative effects on most tumor cells. These results indicate that the bioactivities of sea urchin extracts depend on the part of the urchin that the extract was taken from as well as the extraction method used.



Similar content being viewed by others
References
Kelly MS (2005) Echinodermata. Springer, Heidelberg, pp 139–165
Lawrence JM, Yokota Y (2007) Ecology of Tripneustes. In: Lawrence JM (ed) Edible sea urchin: biology and ecology. Elsevier, Amsterdam, pp 499–520
Dworjanyn SA, Pirozzi I, Liu W (2007) The effect of the addition of algae feeding stimulants to artificial diets for the sea urchin Tripneustes gratilla. Aquaculture 273:624–633
Harnedy PA, FitzGerald RJ (2012) Bioactive peptides from marine processing waste and shellfish: a review. J Funct Foods 4:6–24
Mayer AMS, Rodríguez AD, Berlinck RGS, Fusetani N (2011) Marine pharmacology in 2007–8: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp Biochem Physiol C 153:191–222
Sawai J, Satoh M, Horikawa M, Shiga H, Kojima H (2001) Heated scallop-shell powder slurry treatment of shredded cabbage. J Food Protect 64:1579–1583
Yang JI, Ho HY, Chu YJ, Chow CJ (2008) Characteristic and antioxidant activity of retorted gelatin hydrolysates from cobia (Rachycentron canadum) skin. Food Chem 110:128–136
Bryan PJ, Rittschof D, McClintock JB (1996) Bioactivity of echinoderm ethanolic body-wall extracts: an assessment of marine bacterial attachment and macroinvertebrate larval settlement. J Exp Mar Biol Ecol 196:79–96
Haug T, Kjuul AK, Styrvold OB, Sandsdalen E, Olsen ØM, Stensvåg K (2002) Antibacterial activity in Strongylocentrotus droebachiensis (Echinoidea), Cucumaria frondosa (Holothuroidea), and Asterias rubens (Asteroidea). J Invertebr Pathol 81:94–102
Kim SK, Wijesekara I (2010) Development and biological activities of marine-derived bioactive peptides: a review. J Funct Foods 2:1–9
Sperstad SV, Haug T, Blencke HM, Styrvold OB, Li C, Stensvåg K (2011) Antimicrobial peptides from marine invertebrates; challenges and perspectives in marine antimicrobial peptide discovery. Biotech Adv 29:519–530
Arizza V, Giaramita FT, Parrinello D, Cammarata M, Parrinello N (2007) Cell cooperation in coelomocyte cytotoxic activity of Paracentrotus lividus coelomocytes. Comp Biochem Physiol A 147:389–394
Li C, Blencke HM, Smith LC, Karp MT, Stensvåg K (2010) Two recombinant peptides, SpStrongylocins 1 and 2, from Strongylocentrotus purpuratus, show antimicrobial activity against gram-positive and gram negative bacteria. Dev Comp Immunol 34:286–292
Mamelona J, Pelletier É, Girard-lalancette K, Legault J, Karboune S, Kermasha S (2011) Antioxidants in digestive tracts and gonads of green sea urchin (Strongylocentrotus droebachiensis). J Food Comp Anal 24:179–183
Sahara H, Hanashima S, Yamazaki T, Takahashi S, Sugawara F, Ohtani S, Ishikawa M, Mizushina Y, Ohta K, Shimozawa K, Gasa S, Jimbow K, Sakaguchi K, Sato N, Takahashi N (2002) Anti-tumor effect of chemically synthesized sulfolipids based on sea urchin’s natural sulfonoquinovosylmonoacylglycerols. Jpn J Cancer Res 93:85–92
Schillaci D, Arizza V, Parrinello N, Stefano VD, Fanara S, Muccilli V, Cunsolo V, Haagensen JJA, Molin S (2010) Antimicrobial and antistaphylococcal biofilm activity from the sea urchin Paracentrotus lividus. J Appl Microbiol 108:17–24
Amarowicz R, Synowiecki J, Shahidi F (1994) Sephadex LH-20 separation of pigments from shells of red sea urchin (Strongylocentrotus franciscanus). Food Chem 51:227–229
Amarowicz R, Synowiecki J, Shahidi F (2012) Chemical composition of shells from red (Strongylocentrotus franciscanus) and green (Strongylocentrotus droebachiensis) sea urchin. Food Chem 133:822–826
Li DM, Zhou DY, Zhu BW, Miao L, Qin L, Dong XP, Wang XD, Murata Y (2013) Extraction, structural characterization and antioxidant activity of polyhydroxylated 1,4-naphthoquinone pigments from spines of sea urchin Glyptocidaris crenulars and Strongylocentrotus intermedius. Eur Food Res Technol 237:331–339. doi:10.1007/s00217-013-1996-8
Oktay M, Gülçin İ, Küfrevioğlu Öİ (2003) Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebenson Wiss Technol 36:263–271
Mamelona J, Pelletier É, Girard-Lalancette K, Legault J, Karboune S, Kermasha S (2007) Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chem 104:1040–1047
Shimada K, Fujikawa K, Yahara K, Nakamaru T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948
Dinis TC, maderia VM, Almeida LM (1994) Action of phenolic derivatives (acetaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315:161–169
Oyaizu M (1986) Studies on product of browning reactions: antioxidant activities of products of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Re R, Pellegrini R, Proteggente N, Pannala A, Yang A, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Gimenez B, Aleman A, Montero P, Gomez-Guilen MC (2009) Antioxidant and functional properties of gelatin hydrolysate obtained from skin of sole and squid. Food Chem 114:976–983
Torita A, Miyamoto A, Hasegawa Y (2007) The effects of scallop shell extract on collagen synthesis. Fish Sci 73:1388–1394
Li C, Haug T, Styrvoid OB, Jørgensen TØ, Stensvåg K (2008) Strongylocins, novel antimicrobial peptides from the green sea urchin, Strongylocentrotus droebachiensis. Dev Comp Immunol 32:1430–1440
Kuwahara R, Hatate H, Yuki T, Murata H, Tanaka R, Hama Y (2009) Antioxidant property of polyhydroxylated naphthoquinone pigments from shells of purple sea urchin Anthocidaris crassispina. LWT Food Sci Technol 42:1296–1300
Qin L, Zhu BW, Zhou DY, Wu HT, Tan H, Yang JF, Li DM, Dong XP, Murata Y (2011) Preparation and antioxidant activity of enzymatic hydrolysates from purple sea urchin (Strongylocentrotus nudus) gonad. LWT Food Sci Technol 44:1113–1118
Stabili L, Pagliara P, Roch P (1996) Antibacterial activity in the coelomocytes of the sea urchin Paracentrotus lividus. Comp Biochem Physiol B 113:639–644
Zhou DY, Qin L, Zhu BW, Wang XD, Tan H, Yang JF, Li DM, Dong XP, Wu HT, Sun LM, Li XL, Murata Y (2011) Extraction and antioxidant property of polyhydroxylated naphthoquinone pigments from spines of purple sea urchin Strongylocentrotus nudus. Food Chem 129:1591–1597
Balboa EM, Conde E, Moure A, Falqué E, Domínguez H (2013) In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem 138:1764–1785
Walker CW, Harrington LM, Lesser MP, Fagerberg WR (2005) Nutritive phagocyte incubation chambers provide a structural and nutritive microenvironment for germ cells of Strongylocentrotus droebachiensis, the green sea urchin. Biol Bull 209:31–48
Sheean PD, Hodges LD, Kalafatis N, Wright PF, Wynne PM, Whitehouse MW, Macride TA (2007) Bioactivity of extracts from gonadal tissue of the edible Australian purple sea urchin Heliocidaris erythrogramma. J Sci Food Agric 87:694–701
Kiokias S, Varzakas T (2014) Activity of flavonoids and β-carotene during the auto-oxidative deterioration of model food oil-in-water emulsions. Food Chem 150:280–286
Mamelona J, Pelletier É (2010) Producing high antioxidant activity extracts from echinoderm by products by using pressured liquid extraction. Biotechnology 9:523–528
Anderson HA, Mathieson JW, Thomson RH (1969) Distribution of spinochrome pigments in echinoids. Comp Biochem Physiol 28:333–345
Lamare M, Hoffman J (2004) Natural variation of carotenoids in the eggs and gonads of the echinoid genus, Strongylocentrotus: implications for their role in ultraviolet radiation photoprotection. J Exp Mar Biol Ecol 312:215–233
Ito S, Wakamatsu K, Ozeki H (2000) Chemical analysis of melanins and its application to the study of the regulation of melanogenesis. Pigment Cell Res 13:103–109
Oishi Y, Fu ZW, Ohnuki Y, Kato H, Noguchi T (2002) Molecular basis of the alteration in skin collagen metabolism in response to in vivo dexamethasone treatment: effects on the synthesis of collagen type I and III, collagenase, and tissue inhibitors of metalloproteinases. Br J Dermatol 147:859–868
Torita A, Miyamoto A, Ishiguro K, Yamamoto S, Hasegawa Y (2011) Organic components from scallop shell increase expression of keratinocyte growth factor in human skin fibroblast. Fish Sci 77:263–269
Li C, Blencke HM, Haug T, Jørgensen Ø, Stensvåg K (2014) Expression of antimicrobial peptides in coelomocytes and embryos of the green sea urchin (Strongylocentrotus droebachiemsis). Dev Comp Immunol 43:106–113
Tsushima M, Matsuno T (1997) Occurrence of 9′Ζ-β-echinenone in the sea urchin Pseudocentrotus depressus. Comp Biochem Physiol B 118:921–925
Kawakami T, Tsushima M, Katabami Y, Mine M, Ishida A, Matsuno T (1998) Effect of β,β-carotene, β-echinenone, astaxanthin, fucoxanthin, vitamin A and vitamin E on the biological defense of the sea urchin Pseudocentrotus depressus. J Exp Mar Biol Ecol 226:165–174
Symonds RC, Kelly MS, Caris-Veyrat C, Young AJ (2007) Carotenoids in the sea urchin Paracentrotus lividus: occurrence of 9′-cis-echinenine as the dominant carotenoid in gonad colour determination. Comp Biochem Physiol B 148:432–444
Palozza P, Calviello G, Serini S, Maggiano N, Lanza P, Ranelletti FO, Bartoli GM (2001) β-Carotene at high concentrations induces apoptosis by enhancing oxy-radical production in human adenocarcinoma cells. Free Radic Biol Med 30:1000–1007
Guruvayoorappan C, Kuttan G (2007) β-Carotene down-regulates inducible nitric oxide synthase gene expression and induces apoptosis by suppressing bcl-2 expression and activating caspase-3 and p53 genes in B16F-10 melanoma cells. Nutr Res 27:336–342
Karamanos NK, Manouras A, Anagnostides S, Makatsori E, Tsegenidis T, Antonopoulos CA (1996) Isolation, biochemical and immunological characterization of two sea urchin glycoproteins bearing sulphated poly(sialic acid) polysaccharides rich in N-glycolyl neuraminic acid. Biochimie 78:171–182
Scott LB, Lannarz WJ (1989) Structure of a major yolk glycoprotein and its processing pathway by limited proteolysis are conserved in echinoids. Dev Biol 132:91–102
Brooks JM, Wessel GM (2002) The major yolk protein in sea urchin is a transferrin-like, iron binding protein. Dev Biol 245:1–12
Brooks JM, Wessel GM (2003) Selective transport and packaging of the major yolk protein in the sea urchin. Dev Biol 261:353–370
Unuma T, Ikeda K, Yamano K, Moriyama A, Otha H (2007) Zinc-binding property of the major yolk protein in the sea urchin—implications of its role as zinc transporter for gametogenesis. FEBS J 274:4985–4998
Unuma T, Konishi K, Kiyomoto M, Matranga V, Yamano K, Ohta H, Yokata Y (2009) The major yolk protein is synthesized in the digestive tract and secreted into the body cavities in sea urchin larvae. Mol Reprod Dev 76:142–150
Acknowledgments
This study was supported by funding from the Center of Excellence for Marine Science, National Taiwan Ocean University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, YC., Hwang, DF. Evaluation of antioxidant properties and biofunctions of polar, nonpolar, and water-soluble fractions extracted from gonad and body wall of the sea urchin Tripneustes gratilla . Fish Sci 80, 1311–1321 (2014). https://doi.org/10.1007/s12562-014-0808-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-014-0808-9