Abstract
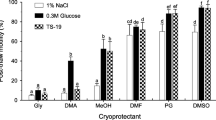
Reliable techniques for the cryopreservation of both sperm and oocytes of the blue mussel Mytilus galloprovincialis Lamarck would increase the availability of seed supplies out-of-season and enhance efficiency in selective breeding. We have investigated the optimal cryo-technique for blue mussel oocytes. The toxicity of three cryoprotective agents (CPAs) [dimethyl sulfoxide (DMSO), ethylene glycol (EG) and propylene glycol (PG)] at different concentrations (1–5 M) and exposure times (0.25–30 min) were investigated for mussel oocytes at room temperature (20 °C) or on ice. The same CPAs (1, 1.5 and 2 M) as well as three different cryoprotectant mixtures [1.5 M EG + 0.2 M trehalose + 100 % Milli-Q water (EGTM); 1.5 M EG + 0.2 M trehalose + 75 % Milli-Q water + 25 % seawater; 1.5 M EG + 0.2 M sucrose + 100 % Milli-Q water] were tested by comparing the post-thaw oocyte fertilization rate after using the slow-cooling method. Vitrification was also examined; however, this method failed to produce any post-thaw surviving oocytes. Among the tested CPAs, EG was the least toxic to oocytes. There was a tendency for the equilibration of CPAs on ice to achieve a higher oocyte fertilization rate compared with that at room temperature, and this difference was significant at concentrations of 3 and 4 M (P < 0.01). The DMSO, EG and PG treatments all resulted in post-thaw fertilization, with EGTM achieving the highest number of surviving oocytes (32 %). At the optimal seeding temperature (−7 °C), the addition of 0.2 M trehalose to EG resulted in a better fertilization rate of post-thawed oocytes than the addition of 0.2 M sucrose. All of the treatments evaluated produced D-larvae from post-thawed oocytes, although the rates were low.





Similar content being viewed by others
References
Whittingham DG, Leibo SP, Mazur P (1972) Survival of mouse embryos frozen to −196° and −269°C. Science 178:411–414
Chen SL, Tian YS (2005) Cryopreservation of flounder (Paralichthys olivaceus) embryos by vitrification. Theriogenology 63:1207–1219
Edashige K, Valdez DMJ, Hara T, Saida N, Seki S, Kasai M (2006) Japanese flounder (Paralichthys olivaceus) embryos are difficult to cryopreserve by vitrification. Cryobiology 53:96–106
Gwo JC (1995) Cryopreservation of oyster (Crassostrea gigas) embryos. Theriogenology 43:1163–1174
Wang HR, Li XX, Wang MQ, Clarke S, Gluis M, Zhang ZY (2011) Effects of larval cryopreservation on subsequent development of the blue mussels, Mytilus galloprovincialis Lamarck. Aquac Res 42:1816–1823
Choi YH, Chang YJ (2003) The influence of cooling rate, developmental stage, and the addition of sugar on the cryopreservation of larvae of the pearl oyster Pinctada fucata martensii. Cryobiology 46:190–193
Chao NH, Lin TT, Chen YJ, Hsu HW, Liao IC (1997) Cryopreservation of late embryos and early larvae in the oyster and hard clam. Aquaculture 155:31–44
Paniagua-Chavez CG, Buchanan JT, Supan JE, Tiersch TR (1998) Settlement and growth of eastern oysters produced from cryopreserved larvae. Cryo-Letters 19:283–292
Chao NH, Liao IC (2001) Cryopreservation of finfish and shellfish gametes and embryos. Aquaculture 197:161–189
Paredes E, Adams SL, Tervit HR, Smith JF, McGowan LT, Gale SL, Morrish JR, Watts E (2012) Cryopreservation of greenshell mussel (Perna canaliculus) trochophore larvae. Cryobiology 65:256–262
Tervit HR, Adams SL, Roberts RD, McGowan LT, Pugh PA, Smith JF, Janke AR (2005) Successful cryopreservation of Pacific oyster (Crassostrea gigas) oocytes. Cryobiology 51:142–151
Hamaratoglu F, Eroglu A, Toner M, Sadler KC (2005) Cryopreservation of starfish oocytes. Cryobiology 50:38–47
Adams SL, Tervit HR, Mcgowan LT, Smith JF, Roberts R, Salinas-Flores L, Gale SL, Webb SC, Mullen SF, Critser JK (2009) Towards cryopreservation of greenshell mussel (Perna canaliculus) oocytes. Cryobiology 58:69–74
Rall WF, Fahy GM (1985) Ice-free cryopreservation of mouse embryos at −196 °C by vitrification. Nature 313:573–575
Leibo SP (1980) Water permeability and its activation energy of fertilized and unfertilized mouse ova. J Membr Biol 53:179–188
Chao NH, Chiang CP, Hsu HW, Tsai CT, Lin TT (1994) Toxicity tolerance of oyster embryos to selected cryoprotectants. Aquat Living Resour 7:99–104
Newton SS, Subramoniam T (1996) Cryoprotectant toxicity in penaeid prawn embryos. Cryobiology 33:172–177
Cabrita E, Robles V, Chereguini O, Wallace JC, Herraez MP (2003) Effect of different cryoprotectants and vitrificant solutions on the hatching rate of turbot embryos (Scophthalmus maximus). Cryobiology 47:204–213
Zhang YZ, Zhang SC, Liu XZ, Xu YJ, Hu JH, Xu YY, Li J, Chen SL (2005) Toxicity and protective efficiency of cryoprotectants to flounder (Paralichthys olivaceus) embryos. Theriogenology 63:763–773
Cabrita E, Robles V, Wallace JC, Sarasquete MC, Herraez MP (2006) Preliminary studies on the cryopreservation of gilthead seabream (Sparus aurata) embryos. Aquaculture 251:245–255
Ding FH, Xiao ZZ, Li J (2007) Preliminary studies on the vitrification of red sea bream (Pagrus major) embryos. Theriogenology 68:702–708
Cortney LO, Andrew LR, Erik S (2009) Viability of subitaneous eggs of the copepod, Acartia tonsa (Dana), following exposure to various cryoprotectants and hypersaline water. Aquaculture 287:114–119
Xiao ZZ, Zhang LL, Xu XZ, Liu QH, Li J, Ma DY, Xu SH, Xue YP, Xue QZ (2008) Effect of cryoprotectants on hatching rate of red seabream (Pagrus major) embryos. Theriogenology 70:1086–1092
Gosling EG (1992) The mussel Mytilus: ecology, physiology, genetics, and culture. Elsevier, Amsterdam
Zhang FS (1984) Mussel culture in China. Aquaculture 39:1–10
Lassen J, Kortegard M, Riisgard HU, Friedrichs M, Graf G, Larsen PS (2006) Down-mixing of phytoplankton above filter-feeding mussels—interplay between water flow and biomixing. Mar Ecol Prog Ser 314:77–88
Soto S, Mena G (1999) Filter feeding by the freshwater mussel, Diplodon chilensis, as a biocontrol of salmon farming eutrophication. Aquaculture 171:65–81
Liu BZ, Li XX (2008) Preliminary studies on cryopreservation of Sydney rock oyster (Saccostrea glomerata) larvae. J Shellfish Res 27:1125–1128
Fujino Y, Kojima T, Nakamura Y, Kobayashi H, Kikuchi K, Funahashi H (2008) Metal mesh vitrification (MMV) method for cryopreservation of porcine embryos. Theriogenology 70:809–817
Maclellan LJ, Carnevale EM, Coutinho da Silva MA, Scoggin CF, Bruemmer JE, Squires EL (2002) Pregnancies from vitrified equine oocytes collected from super-stimulated and non-stimulated mares. Theriogenology 58:911–919
Rojas C, Palomo MJ, Albarracin JL, Mogas T (2004) Vitrification of immature and in vitro matured pig oocytes: study of distribution of chromosomes, microtubules, and actin microfilaments. Cryobiology 49:211–220
Lin TT, Chao NH, Tung HT (1999) Factors affecting survival of cryopreserved oyster (Crassostrea gigas) embryos. Cryobiology 39:192–196
Stachecki JJ, Cohen J, Willadsen SM (1998) Cryopreservation of unfertilized mouse oocytes: the effect of replacing sodium with choline in the freezing medium. Cryobiology 37:346–354
Özkavukcu S, Erdemli E (2002) Cryoperservation: basic knowledge and biophysical effects. J Ankara Med Sch 24:187–196
Arellano SM, Young CM (2009) Spawning, development, and the duration of larval life in a deep-sea cold-seep mussel. Biol Bull 216:149–162
Lango-Reynoso F, Chavez-Villalba J, Cochard JC, Le Pennec M (2000) Oocyte size, a means to evaluate the gametogenic development of the Pacific oyster, Crassostrea gigas (Thunberg). Aquaculture 190:183–199
Hamano S, Koikeda A, Kuwayama M, Nagai T (1992) Full-term development of in vitro-matured, vitrified and fertilized bovine oocytes. Theriogenology 38:1085–1090
Lin TA, Chen CH, Sung LY, Carter MG, Chen YE, Du F, Ju JC, Xu J (2011) Open-pulled straw vitrification differentiates cryotolerance of in vitro cultured rabbit embryos at the eight-cell stage. Theriogenology 75:760–768
Acknowledgments
The authors would like to thank Assoc. Prof. David Stone and Dr. Ron Smernik for their assistance in proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Li, X., Wang, M. et al. The development of oocyte cryopreservation techniques in blue mussels Mytilus galloprovincialis . Fish Sci 80, 1257–1267 (2014). https://doi.org/10.1007/s12562-014-0796-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-014-0796-9