Abstract
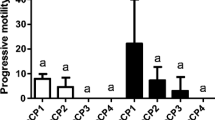
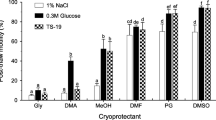
In this study, a programmable freezing technique has been developed for strip spawned sperm in the blue mussel, Mytilus galloprovincialis. The optimized key parameters include cooling rate, endpoint temperature, thawing temperature, sugar addition and sperm to oocyte ratio. The sperm quality was assessed by the fertilization rate or the integrity of sperm component and organelle. The highest post-thaw sperm fertilization rate was 91%, which was produced with sperm cryopreserved in 8% dimethyl sulfoxide at the cooling rate of -4°C/min from 2°C to -30°C before being plunged into liquid nitrogen for at least 12 h, thawed in a 20°C seawater bath and fertilized at sperm to egg ratio of 50 000:1. The addition of glucose, sucrose or trehalose to 8% dimethyl sulfoxide could not further improve fertilization rates. The fluorescent assessments showed that the post-thaw sperm plasma membrane integrity and acrosome integrity were significantly damaged in comparison with fresh sperm.
Similar content being viewed by others
References
Adams S L, Smith J F, Roberts R D, Janke A R, Kaspar H F, Tervit H R, Pugh P A, Webb S C, King N G. 2004. Cryopreservation of sperm of the Pacific oyster ( Crassostrea gigas ): development of a practical method for commercial spat production. Aquaculture, 242 (1–4): 271–282.
Anchordoguy T, Crowe J H, Griffin F J, Clark W H. 1988. Cryopreservation of sperm from the marine shrimp sicyonia ingentis. Cryobiology, 25 (3): 238–243.
Cabrita E, Sarasquete C, Martínez–Páramo S, Robles V, Beirão J, Pérez–Cerezales S, Herráez M P. 2010. Cryopreservation of fish sperm: applications and perspectives. Journal of Applied Ichthyology, 26 (5): 623–635.
Clulow J R, Mansfield L J, Morris L H A, Evans G, Maxwell W M C. 2008. A comparison between freezing methods for the cryopreservation of stallion spermatozoa. Animal Reproduction Science, 108 (3–4): 298–308.
Di Matteo O, Langellotti A L, Masullo P, Sansone G. 2009. Cryopreservation of the Mediterranean mussel ( Mytilus galloprovincialis ) spermatozoa. Cryobiology, 58 (2): 145–150.
Dong Q X, Eudeline B, Huang C J, Allen S K, Tiersch T R. 2005. Commercial–scale sperm cryopreservation of diploid and tetraploid pacific oysters, Crassostrea gigas. Cryobiology, 50 (1): 1–16.
Dupré E, Guerrero A. 2011. Cryopreservation of Macha surf clam spermatozoa. in: Tiersch T R, Green C C eds. Cryopreservation in Aquatic Species. 2 nd edn. World Aquaculture Society, Baton Rouge. p.574–580.
Gao D Y, Critser J K. 2000. Mechanisms of cryoinjury in living cells. ILAR Journal, 41 (4): 187–196.
Gómez–Fernández J, Gómez–Izquierdo E, Tomás C, Mocé E, De Mercado E. 2012. Effect of different monosaccharides and disaccharides on boar sperm quality after cryopreservation. Animal Reproduction Science, 133 (1–2): 109–116.
Gwo J C, Chen C W, Cheng H Y. 2002. Semen cryopreservation of small abalone ( Haliotis diversicolor supertexa ). Theriogenology, 58 (8): 1 563–1 578.
Gwo J C. 2008. Cryopreservation of small abalone ( Haliotis diversicolor supertexa ) semen. in: Cabrita E, Robles V, Herráez P eds. Methods in Reproductive Aquaculture: Marine and freshwater Species. CRC Press, New York. 480p.
Hassan M M, Qin J G, Li X X. 2015. Sperm cryopreservation in oysters: a review of its current status and potentials for future application in aquaculture. Aquaculture, 438: 24–32.
Herráez P, Cabrita E, Robles V. 2012. Fish gamete and embryo cryopreservation: state of the art. in: Fletcher G L, Rise M L eds. Aquaculture Biotechnology. Wiley–Blackwell, Oxford, UK. p.303–317.
Hopkins B K, Herr C. 2010. Factors affecting the successful cryopreservation of honey bee ( Api s mellifera ) spermatozoa. Apidologie, 41 (5): 548–556.
Ieropoli S, Masullo P, Santo M D E, Sansone G. 2004. Effects of extender composition, cooling rate and freezing on the fertilisation viability of spermatozoa of the Pacific oyster ( Crassostrea gigas ). Cryobiology, 49 (3): 250–257.
Jafaroghli M, Khalili B, Farshad A, Zamiri M J. 2011. The effect of supplementation of cryopreservation diluents with sugars on the post–thawing fertility of ram semen. Small Ruminant Research, 96 (1): 58–63.
Kang K H, Kim J M, Kim Y H. 2004. Short–term storage and cryopreservation of abalone ( Haliotis discus hannai ) sperm. The Korean Journal of Malacology, 20 (1): 17–26.
Kawamoto T, Narita T, Isowa K, Aoki H, Hayashi M, Komaru A, Ohta H. 2007. Effects of cryopreservation methods on post–thaw motility of spermatozoa from the Japanese pearl oyster, Pinctada fucata martensii. Cryobiology, 54 (1): 19–26.
Lazo C S, Pita I M. 2012. Effect of temperature on survival, growth and development of Mytilus galloprovincialis larvae. Aquaculture Research, 43 (8): 1 127–1 133.
Li C, Li J, Xue Q Z. 2000. Cryopreservation of the spermatozoa of Chlamys ( Azumapecten ) farreri. Marine Fisheries Research, 21 (1): 57–62. (in Chinese with English abstract)
Liu B L, Liu Y B, Liu S W, Xu T, Liu Q, Li X X. 2016a. Cryopreservation of strip spawned sperm using nonprogrammable freezing technique in the blue mussel Mytilus galloprovincialis. Aquaculture Research, 47 (12): 3 888–3 898.
Liu Y B, Li X X, Robinson N, Qin J G. 2015a. Sperm cryopreservation in marine mollusk: a review. Aquaculture International, 23 (6): 1 505–1 524.
Liu Y B, Li X X, Xu T, Robinson N, Qin J G. 2014a. Improvement in non–programmable sperm cryopreservation technique in farmed greenlip abalone Haliotis laevigata. Aquaculture, 434: 362–366.
Liu Y B, Li X X, Xu T, Robinson N, Qin J G. 2016b. Greenlip abalone ( Haliotis laevigata Donovan, 1808) sperm cryopreservation using a programmable freezing technique and testing the addition of amino acid and vitamin. Aquaculture Research, 47 (5): 1 499–1 510.
Liu Y B, Xu T, Robinson N, Qin J G, Li X X. 2014b. Cryopreservation of sperm in farmed Australian greenlip abalone Haliotis laevigata. Cryobiology, 68 (2): 185–193.
Liu Y B, Xu T, Robinson N, Qin J G, Li X X. 2015b. Cryopreservation of sperm in farmed blacklip abalone ( Haliotis rubra Leach, 1814). Aquaculture Research, 46 (11): 2 628–2 636.
Lyons L, Jerry D R, Southgate P C. 2005. Cryopreservation of black–lip pearl oyster ( Pinctada margaritifera, L.) spermatozoa: effects of cryoprotectants on spermatozoa motility. Journal of Shellfish Research, 24 (4): 1 187–1 190.
Paniagua–Chavez C G, Buchanan J T, Tiersch T R. 1998. Effect of extender solutions and dilution on motility and fertilizing ability of eastern oyster sperm. Journal of Shellfish Research, 17: 231–237.
Pettersen A K, Turchini G M, Jahangard S, Ingram B A, Sherman C D H. 2010. Effects of different dietary microalgae on survival, growth, settlement and fatty acid composition of blue mussel ( Mytilus galloprovincialis ) larvae. Aquaculture, 309 (1–4): 115–124.
Purdy P H. 2006. A review on goat sperm cryopreservation. Small Ruminant Research, 63 (3): 215–225.
Rota A, Rota A, Martini M, Milani C, Romagnoli S. 2005. Evaluation of dog semen quality after slow (biological freezer) or rapid (nitrogen vapours) freezing. Reproduction Nutrition Development, 45 (1): 29–37.
Salinas–Flores L, Paniagua–Chavez C G, Jenkins J A, Tiersch T R. 2005. Cryopreservation of sperm of red abalone ( Haliotis rufescens ). Journal of Shellfish Research, 24 (2): 415–420.
Stanic P, Tandara M, Sonicki Z, Simunic V, Radakovic B, Suchanek E. 2000. Comparison of protective media and freezing techniques for cryopreservation of human semen. European Journal of Obstetrics & Gynecology and Reproductive Biology, 91 (1): 65–70.
Suquet M, Dreanno C, Fauvel C, Cosson J, Billard R. 2000. Cryopreservation of sperm in marine fish. Aquaculture Research, 31 (3): 231–243.
Tiersch T R. 2008. Strategies for commercialization of cryopreserved fish semen. Revista Brasileira de Zootecnia, 37 suplemento especial: 15–19.
Vitiello V, Carlino P A, Del Prete F, Langellotti A L, Sansone G. 2011. Effects of cooling and freezing on the motility of Ostrea edulis (L., 1758) spermatozoa after thawing. Cryobiology, 63 (2): 118–124.
Viveiros A T M, Lock E J, Woelders H, Komen J. 2001. Influence of cooling rates and plunging temperatures in an interrupted slow–freezing procedure for semen of the African catfish, Clarias gariepinus. Cryobiology, 43 (3): 276–287.
Zhang X M, Li X X, Clarke S, Li X. 2012. The development of Pacific oysters Crassostrea gigas produced using cryopreserved sperm. In: Qin J G ed. Oysters: Physiology, Ecological Distribution and Mortality. Nova Science, New York. p.1–18.
Acknowledgement
We thank Mr Andy Dyle for the provision of blue mussel broodstock and Mr Mark Gluis of SARDI for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the China Scholarship Council and South Australian Research and Development Institute (SARDI)
Rights and permissions
About this article
Cite this article
Liu, Y., Liu, S., Liu, B. et al. Cryopreservation of strip spawned sperm using programmable freezing technique in the blue mussel Mytilus galloprovincialis. J. Ocean. Limnol. 36, 2351–2357 (2018). https://doi.org/10.1007/s00343-019-7252-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-019-7252-8