Abstract
This study reviews the present status of the Japanese chum salmon Oncorhynchus keta stock enhancement program and considers the ecological sustainability of wild populations while providing fishery production, exemplified by the hatchery-based Kitami region set net fishery. The return rate and the number of returns have been historically high in the Sea of Okhotsk, but have decreased in other regions since 2005. Natural spawning of chum salmon occurred in at least 160 rivers in Hokkaido. The genetic diversity of Japanese chum salmon was similar to or higher than that of other Pacific Rim populations. Numbers of alleles were high at microsatellite loci, but the loss of rare haplotypes was observed in all populations. The estimated N e /N ratio for the Kitami region was >0.15 % including hatchery and wild fish under the present high fishing pressure. Four regional populations were inferred in Hokkaido, however, genetic differentiation was weak and some river-populations were nested. Substantial changes in run timing were observed, but it has recovered gradually owing to the recent practice of escapement. Our analyses highlight the importance of juvenile quality and the vital roles of escapements in enhanced and non-enhanced rivers. New research is needed to minimize the genetic risks associated with hatchery programs.
Similar content being viewed by others
Introduction
The hatchery stock enhancement program in Japan was initiated in the 1880s to increase Japanese chum salmon Oncorhynchus keta fishery production and has continued for over 120 years. The numbers of chum salmon returning to Japan have increased remarkably since the late 1970s. Hatchery technology innovation is considered the major cause for the increase [1, 2], and the favorable ocean and climate conditions have supported this increase [3, 4]. The closure of high seas fisheries and improved river water quality may have boosted the recent abundance of chum salmon in Hokkaido [5], the principal salmon production area in Japan. Although past management of Japanese chum salmon focused on producing and releasing hatchery-reared fish (hereafter hatchery fish), Japanese salmon scientists and hatchery managers have become aware of the importance of conserving wild fish. Counting surveys of wild fish recently conducted in Hokkaido have found that natural spawning occurs in many rivers [6]. However, the majority of chum salmon returning to Hokkaido are hatchery-released fish; therefore, the ecological sustainability of the chum salmon stock is controversial [7–9]. While traditional hatcheries have concentrated on fish production, the goals of modern hatcheries are to conserve wild populations and/or provide harvest with minimal risk to the wild population [10, 11]. There are strong ecological and economic incentives in Hokkaido to reform salmon fishery management by focusing on the coexistence of hatchery and wild fish [2, 12–14]. Further discussion on the use of hatcheries from the viewpoint of conservation may be useful.
This study reviews the present status of the Japanese chum salmon stock enhancement program and considers the role of hatcheries in the ecological sustainability of wild populations, focusing on the hatchery-based set net fishery in the Kitami region, Hokkaido, the largest chum salmon fishery in Japan. Onsite surveys of the chum salmon stock enhancement program were conducted, and statistics and information were collected from the Salmon and Freshwater Fisheries Research Institute (SFFRI) at Eniwa, the Hokkaido Federation of Fisheries Cooperative Association (HFFCA) at Sapporo, and the Kitami Salmon Enhancement Program Association (KSEPA) at Abashiri. After conducting a brief review of the chum salmon stock enhancement program and the results of wild fish counting surveys in Hokkaido, the ratio of effective population size to the number of returning adult fish was estimated for the Kitami region by combining hatchery broodstock and hatchery/wild escapements. Genetic diversity indices of Japanese chum salmon populations were compared with those of other populations in the Pacific Rim. Fine-scale population structures of Japanese chum salmon populations were estimated based on publicly available mitochondrial (mt) DNA and microsatellite (ms) DNA data. Our analyses highlight the importance of juvenile fish quality and the vital role of hatchery-wild integrated reproduction through escapements to natural streams.
Hatchery releases and return rates to Hokkaido
The chum salmon hatchery program in Hokkaido is one of the largest salmon hatchery programs in the world [2]. The number of juveniles released from Hokkaido has been stable since the 1990s, averaging 1,030 ± 22 (standard deviation) million per annum during the last decade. There are currently 150 private and 10 national hatcheries in Hokkaido, and the majority of the chum salmon released (87 %) are produced by private hatcheries, with the residual portion (13 %) being produced and released by national hatcheries [2]. Hatchery fish are released in 140 rivers, of which 60 rivers use juveniles produced from parent fish collected in adjacent rivers (transplants). In addition, transplants are reared in 80 net pens and released from the rearing sites [2]. Such transplants are allowed within the geographic region in order to minimize genetic disturbance. All juveniles released from national hatcheries have otolith thermal marks. Fish from private hatcheries are marked only when scientific research is conducted. The otolith-marked chum salmon are currently <15 % of the total number released [2].
The number of chum salmon returning to Hokkaido has increased remarkably since the late 1970s, and the average number was 49 ± 10 million (range 38–61 million) during 2008–2012. This number has been decreasing since 2005, and as of 2012 (39 million) was ~64 % of the historical maximum of 61 million in 2004 (Fig. 1a). The average return rate was ~5 % for the brood years after 1996 [2]. In this article, the return rate was simply calculated by the number of returns (i.e., coastal commercial catches plus the number of adults caught for broodstock in rivers) divided by the number of hatchery fish released in the previous 4 years with the assumption that the number of naturally spawned chum salmon was negligible if any [2]. Returning salmon are caught by set net fisheries in the coastal waters off Hokkaido. Approximately 1,000 salmon set nets are operated from late August to late December, with landings peaking in late September and early October, although the fishing season differs among regions [14]. Commercial fishers pay for ~7 % of the landings every year to support the hatchery program. Commercial and recreational fishing for adult chum salmon are prohibited in all rivers and in coastal waters near the river mouths where hatchery broodstock are collected. Therefore, chum salmon that have escaped capture by commercial set nets are only captured at weirs at enhanced rivers [2]. Surplus fish caught in weirs are sold to support hatchery operations.
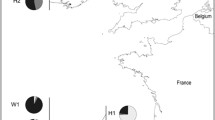
Numbers of releases and returns with return rates of chum salmon in a total, and in the regions of the b Sea of Okhotsk coast (Kitami region), c Nemuro Strait, d eastern Pacific, e western Pacific, and f Sea of Japan, Hokkaido. The return rate was estimated as (number returned 4 years after release/number released)
The Hokkaido Prefectural Government established the salmon enhancement plan in 1999, which prescribes the number of adults to be captured for broodstock, number of eggs to be taken in each river, number of fry to be reared in each hatchery, and the number of fry to be released in each river or net pen location [2]. In the enhancement plan, Hokkaido is divided into five geographical regions (Sea of Okhotsk coast, Nemuro Strait, eastern Pacific coast, western Pacific coast, and Sea of Japan coast) [14], each of which is further subdivided into 2–4 sub-regions, resulting in 14 management regions [2]. The hatcheries are operated by the nine regional salmon enhancement program associations, including KSEPA, which are supported by the Hokkaido Salmon Propagation Association, Sapporo. The salmon enhancement plan is temporally divided into three periods to manage the coastal set net fishery and broodstock collection in rivers [2]. These periods for the coastal fishery include early- (September), middle- (October), and late-periods (November and later). However, this is a period for fishery management, and not for identifying biological differences in returning fish. Here, the term “early run” is tentatively defined for fish that return during September–November, and “late run” for fish that return during December–January. The term “wild fish” is defined as naturally-produced individuals in the wild, even though they could include hatchery descendants [15–17].
A decreasing trend in returns has been observed in all regions except the Sea of Okhotsk. The recent return rates and the number of fish returning have been historically high in the Sea of Okhotsk and relatively low in the Sea of Japan region (Fig. 1). Release sizes have been increasing in all regions, and at most hatcheries they exceed the target size to be reared, i.e., 5 cm in fork length and 1 g in weight [2]. Despite the large release sizes, declining return rates have been observed in the Nemuro Strait, eastern Pacific, and western Pacific regions (Fig. 1). A plausible cause for the regional difference in return rates is the recent changes in the coastal environment around release time, but there remains an insufficient understanding of the causes [2].
The average number of chum salmon returning to the Sea of Okhotsk coast was 1,369,000 during 1959–1970. The average number of returning fish for the past 5 years (2008–2012) was 16,998,000 (12.4 times larger), most of which were hatchery fish. Hatchery innovation techniques are considered the main reason for the remarkable increase, which mimics the wild recruitment process and allows release timing to match favorable conditions [13] in river and coastal areas. In addition, KSEPA, an agency that enhances chum salmon populations in the Sea of Okhotsk region, has improved seed production techniques during the past decade to produce healthy juveniles. The quality of released juveniles may have contributed to the increased number of returning fish and return rates after 2000 (Fig. 1b). It may be useful to evaluate the present management system in the Kitami region and consider chum salmon sustainability, which depends on both hatchery and wild fish.
Natural spawning in non-enhanced and enhanced rivers
About 1,500 river systems occur in Hokkaido, of which 140 (9.3 %) rivers currently receive hatchery-releases of chum salmon [6]. The rivers are categorized into four types in relation to hatchery operations: (1) no enhancement to date, (2) currently no enhancement, (3) enhanced without broodstock collection, and (4) enhanced with broodstock collection for seed production [6, 14]. The rivers are relatively short, so hatchery fish are released in most large rivers with lengths >50 km, and naturally spawning chum salmon populations remain in many small (short) rivers. A total of 322 rivers (length > 8 km) were selected for counting surveys throughout Hokkaido, because water levels in smaller rivers may be inadequate to allow the passage of chum salmon [6]. Therefore, the number of rivers possible for chum salmon runs is estimated to be at least 322 in Hokkaido. Among the rivers, the number of enhanced rivers (categories 3 and 4) was 140 (43.4 %), of which 76 (2008) (23.8 %) and 84 (2009) (26.0 %) had weirs to collect hatchery broodstock (category 4).
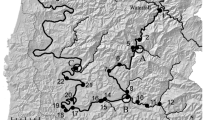
Miyakoshi et al. [6] conducted foot surveys of 238 rivers during 2008 and 2009 and found that natural spawning occurred in 59 (31.4 %) and 50 (37.6 %) of the non-enhanced rivers, respectively. Furthermore, chum salmon were seen to ascend upstream in 191 and 175 rivers, including rivers from which broodstock are collected (76 and 84 rivers) and rivers <8 km (11 and eight rivers), respectively. These observations show that natural spawning of chum salmon occurs in 30–40 % of non-enhanced rivers (Fig. 2a) and in 70–80 % of 238 rivers surveyed, including categories 1–4.
The escapement of hatchery fish into enhanced rivers with hatcheries creates wild fish. Escapement during hatchery operations supplements early-run wild fish, and escapement creates late-run wild fish after hatchery operations. All weirs in the enhanced rivers of the Kitami region (Fig. 2b, c) have been remodeled recently to provide passage of fish for spawning (KSEPA, pers. comm., Fig. 2d). KSEPA recorded the number of escapements after 2005 in some enhanced rivers with hatcheries, although the number was low (Fig. 3). Weirs are removed around the end of November, when hatchery operations are finished. Returning hatchery and wild fish freely ascend the stream and spawn in the wild on enhanced rivers, and supplement early and late-run wild fish, respectively. The natural run in enhanced rivers without hatcheries and non-enhanced rivers creates wild fish throughout the run season. Thus, regardless of enhancement, wild fish can be created in all river systems. Therefore, it is important to evaluate how the sustainability of the chum salmon populations is impacted by integrated reproduction in hatcheries and natural streams.
Integrated hatchery and wild reproduction
Huge numbers of hatchery fish return to hatchery-enhanced rivers, mainly in September–November. In 2012, 18,460,000 fish returned to the Kitami region. Among them, ~16 million fish were caught by set nets in coastal areas, and 2 million fish were caught at weirs on enhanced rivers, of which 114,000 fish were sent to hatcheries as parent fish, which provided an estimate that 4,140 hatchery fish escaped upstream for wild stock conservation. The surplus hatchery fish caught in weirs (1,886,000) were sold to support hatchery operations. The exploitation rates were very high at 86.7 % (set nets) and 94.3 % (weirs). This calculation indicates that 0.62 % of returning hatchery fish were used for hatchery broodstock and 0.02 % escaped from weirs to upstream rivers.
Substantial numbers of wild fish return to some Kitami-region rivers [6, 18]. The largest portion of this natural return occurs during December and early January, after returns of hatchery fish and the hatchery-focused fishery are completed in late November. The management system believes that fish returning during this time frame represent the historical natural return and are not subject to significant harvest. Escapements of these fish have been relatively consistent for the last several years, and current wild salmon escapement was estimated at 20,191 in the Kitami region (SFFRI, unpubl. data, 2013).
The percentage of hatchery breeders among all returned fish is very small (0.62 %). However, this is not surprising because the effective population size (N e ) is very small compared with the census population size (N) [19]. It is generally accepted that the effective population size of highly fecund marine teleosts is between two and five orders of magnitude smaller than the census spawning size [20–22]. For example, the N e /N ratio was 10−5 for North Sea cod Gadus morhua [22] and New Zealand snapper Pagrus auratus [23], 10−3 for red drum Sciaenops ocellatus [24], and 10−4 for Japanese Spanish mackerel Scomberomorus niphonius in the Seto Inland Sea [17]. This may be explained by a biased sex ratio caused by dominant males monopolizing females, a disproportionate contribution of genotypes through egg production, an increase in larval viability with increasing female size, overlapping generations, or high variance in individual reproductive success [22]. In addition, the chance spatial and temporal match or mismatch of spawning with suitable oceanic conditions [20, 25] could result in the majority of surviving recruits being spawned by a minority of the population [17, 22].
The N e /N ratio for salmonids should be larger than that of marine fish because of lower fecundity with larger eggs. The N e /N ratio was estimated to be 4.3 % for a natural population of Chinook salmon O. tshawytscha [26]. The N e /N ratio is on the order of 0.2 for most natural stocks of salmonid fish, where N is the number of spawning adults [27]. No N e /N ratio estimate for a natural and a hatchery population of chum salmon was found in the literature, so 20 % (~10−1) was used as a tentative estimate of N e /N for both hatchery and wild spawning chum salmon. The number of spawning adults in hatcheries was 114,000, which was assumed to be hatchery-released fish. The rough estimate of the N e /N ratio for the Kitami hatchery program was ~0.12 % (=114,000 × 0.2/18,460,000 × 100), and ~0.03 % (=24,331 × 0.2/18,460,000 × 100) for the estimated escapement (24,331 = 4,140 hatchery + 20,191 wild fish). The total N e estimate was 27,666 (=(114,000 + 24,331) × 0.2) and the N e /N ratio was ~0.15 % (10−3) after combining the hatchery broodstock and escapement. Current wild salmon escapement (20,191) was ~0.1 % of the 18,460,000 fish returned to the Kitami region in September–November, which was an underestimate because it did not account for naturally spawning salmon in major enhanced rivers (SFFRI, pers. comm.). Therefore, the N e /N ratio estimate (~0.15 %) should be an underestimate. In addition, the N e /N ratio might be much higher in hatcheries, as reported for rainbow trout O. mykiss (~90 %) [26]. A large number of adults (114,000) were used for artificial fertilization and the sex ratio was adjusted to ~0.5. However, even if this was the case, the N e /N ratio of the chum salmon in the Kitami region was very small (~10−2). It may be much smaller than those of Chinook salmon, coho salmon O. kisutch, steelhead trout O. mykiss, and Atlantic salmon Salmo salar, reflecting differences in early life history and the large census population size of chum salmon. To estimate the N e /N ratio correctly, the effective population size of returned fish and the effective number of breeders in hatcheries needs to be estimated based on genetic monitoring [28].
Genetic diversity
Preceding studies on chum salmon using different genetic markers have provided valuable information on population sustainability. Sato et al. [29] examined the genetic population structure of chum salmon in the Pacific Rim using the mtDNA control region for 2,154 individuals from 47 rivers in 15 populations from Japan, Korea, Russia, and North America, and found that haplotype diversity was highest in the Japanese populations. Beacham et al. [30] surveyed genetic variation at 14 microsatellite loci in 3,602 individuals from 26 Japanese chum salmon populations, one population from Kamchatka, and three populations from North America, and found that the Japanese populations are more genetically diverse at each of the 14 microsatellites surveyed than that of North American populations in the Yukon River and British Columbia. Seeb et al. [31] surveyed the allelic variation of 10,458 chum salmon at 60 loci of single-nucleotide polymorphisms (SNPs) in 114 locations from Japan (16 sites), Korea, Russia, and North America, and showed that neither marker type suggested that the Japanese populations are inherently less diverse than those from North America, as suggested by the current SNP panel. Genetic diversity indices of Japanese chum salmon for the three markers were generally higher or similar in comparison with Korean, Russian, and North American chum salmon populations (Fig. 4).
Genetic diversity of chum salmon in the Pacific Rim. K refers to the number of rivers where samples were taken (white: Japan, grey: other countries). a Number of haplotypes for the mitochondrial DNA control region (data from [29], n = 2,154). Error bars show standard deviations. HK Hokkaido, HS Honshu, KR Korea, RUS Russia, NWA northwest Alaska and Alaskan peninsula, SCA south-central Alaska, SEA southeast Alaska, BC British Columbia, WA Washington. b Number of alleles for 14 microsatellite DNA loci (data from [30], n = 3,602). HSJ Honshu, Sea of Japan coast), HSP Honshu, Pacific coast, HKO Hokkaido, Sea of Okhotsk coast, HKN Hokkaido, Nemuro Strait, HKE Hokkaido, eastern Pacific coast, HKW Hokkaido, western Pacific coast, HKJ Hokkaido, Sea of Japan coast, KM Kamchatka, YK Yukon, BC British Columbia. c Number of alleles for 60 single-nucleotide polymorphic loci (data from [31], n = 10,458). 1, Hokkaido; 2, Honshu; 3, Amur; 4, Kamchatka; 5, Alaska; 6, western Alaska; 7, Yukon; 8, Yukon2; 9, Bristol Bay; 10, north Alaskan peninsula; 11, southern Alaskan peninsula; 12, Kodiak Island; 13, Susitna River; 14, Alaska Bay; 15, Washington
Population structure
Analyses of the mtDNA control region and SNPs coincidently show that the Japanese and Korean populations form a single, distinct cluster [29, 31]. Analyses of msDNA [30] found five clusters of populations in Hokkaido (Sea of Okhotsk coast, Nemuro Strait, eastern Pacific coast, western Pacific coast, and Sea of Japan coast) and two in Honshu (Pacific coast and Sea of Japan coast). Honshu Pacific coast had the most distinct cluster. KSEPA and Japanese salmon scientists suggest that the December–January return (late run) represents the native return and that the hatchery-dominated September–November return represents transplants. An analysis of the mtDNA control region from four sites in Hokkaido (n = 323) [32] showed that the Yurappu River chum salmon has two populations: the late native-population spawning in December and the early mixed-population introduced from other river populations in October and November.
To infer the fine population structure including early and late-run populations, genetic differentiation was estimated using a common scale in terms of F ST based on mtDNA haplotype frequencies of 15 samples (n = 273) [29, 32, 33] and msDNA allele frequencies of 26 samples (n = 2,670) at 14 loci [30]. The samples [29, 30, 33] were collected in hatcheries (hatchery-released fish), and the Yurappu sample of Yokotani et al. [32] consisted of naturally-spawning fish (Kaeriyama M. pers. comm.). The msDNA allele frequencies were cited from the web site of the Molecular Genetics Lab at the Pacific Biological Station, Fisheries and Ocean Canada (http://www.pac.dfo-mpo.gc.ca/science/facilities-installations/pbs-sbp/mgl-lgm/data-donnees/index-eng.html). We used an empirical Bayesian method [34] with the software POPDIF 1.0 (http://popgene.co-site.jp/software_eng.html) to avoid a biased estimation of pairwise F ST (e.g., negative F ST values), which is a concern for species with high gene flow such as marine fish. The maximum likelihood global F ST over populations, the rate of gene flow (=1/F ST − 1), and the means of the posterior distributions of pairwise F ST were estimated based on haplotype and allele frequencies.
Global F ST was estimated as 0.0433 ± 0.0130 for the mtDNA haplotype frequencies. The rate of gene flow was 22.1 ± 6.9, suggesting that 22 females in terms of the effective population size migrate between rivers per generation. The unweighted pair group method with arithmetic mean (UPGMA) dendrogram of pairwise F ST showed a distinct cluster in the samples taken in September and October. Honshu Pacific coast samples also formed a cluster. In contrast, samples taken in November and December were included in a different cluster for except Tokushibetsu, collected in September (Fig. 5). The November samples were distinct from the October samples at Tokoro and Yurappu, suggesting that it was influenced by transplantations from the Chitose hatchery. The pairwise F ST value [95 % credible interval] between October and November was small at 0.0160 [0.0013, 0.0482] for Tokoro and 0.0125 [0.0017, 0.0352] for Yurappu, respectively. F ST between November and December for Yurappu was 0.0290 [0.0050, 0.0708] and between October and December was 0.0529 [0.0158, 0.1072]. The genetic difference was greater for the later run. However, the posterior F ST distributions overlapped, clearly showing that early- and late-run populations were not genetically distinct with high gene flow (Fig. 6). The difference between Yurappu (December) and Tokushibetsu (September) was also similar at 0.0349 [0.0084, 0.0750]. It was unclear why the early sample from Tokushibetsu was close to the late one from Yurappu.
Genetic differentiation of chum salmon based on the posterior mean of pairwise F ST, inferred from mitochondrial DNA control region haplotype frequencies of 15 geographical samples (n = 273) (data from Sato et al. [29, 33], Yokotani et al. [32] ). Hokkaido, Sea of Okhotsk Coast (red circle); Hokkaido, Nemuro Strait (yellow circle); Hokkaido, eastern Pacific coast (brown circle); Hokkaido, western Pacific coast (dark green circle); Hokkaido, Sea of Japan coast (purple circle); Honshu, Pacific coast (light green circle); Honshu, Sea of Japan coast (blue circle). S, September; O, October; N, November; D, December; A, April
The global F ST was much smaller (0.0069 ± 0.0002) than that from the mtDNA haplotype frequencies based on the msDNA allele frequencies. The rate of gene flow was 155.3 ± 4.7. The UPGMA dendrogram of pairwise F ST showed three clusters. Honshu Pacific coast samples formed a distinct cluster, coinciding with the results from the mtDNA analysis. Five geographical samples (except from the eastern Pacific coast of Hokkaido) were located closely in the large middle cluster, but some were nested. The Honshu Sea of Japan coast formed one cluster with Teshio and Chitose, but Kawabukuro was close to the western Pacific coast of Hokkaido, and Uono was separated from the cluster. Hokkaido samples from the Sea of Okhotsk, Nemuro Strait, the western Pacific coast, and the Sea of Japan coast were generally located nearby. In contrast, samples from the Pacific coast of Hokkaido (Kushiro and Tokachi) were not close and Horonai was different from other Sea of Okhotsk samples (Fig. 7), though the F ST value between Horonai and Tokoro was very small at 0.0036 [0.0011, 0.0082]. Our results generally coincided with the results of Beacham et al. [30], who found seven regional populations (five on Hokkaido and two on Honshu) within Japan, and learned that the Honshu Pacific coast populations were the most distinct based on Cavalli–Sforza and Edwards chord distance. However, the genetic differentiation was very small and population structure was not rigid in terms of the posterior mean of F ST. The samples were all hatchery fish, as mentioned above. The nested population structure in Hokkaido may have been influenced by the transplantation of hatchery fish. No sample set was available from the same site including both early and late runs, so it was not possible to detect the genetic difference between the two runs. Beacham et al. [30] analyzed the original temporal samples from three populations within 1 year: (1) Gakko River, October and December samples; (2) Yurappu River, September and November samples; and (3) Chitose River, October and December samples. Their F ST values were 0.015 ± 0.002 (Gakko), 0.008 ± 0.002 (Yurappu), and 0.010 ± 0.001 (Chitose). Beacham et al. [30] suggested that transplantation history could influence the observed temporal variation within these populations.
Genetic differentiation of chum salmon based on the posterior mean of pairwise F ST inferred from microsatellite DNA allele frequencies of 26 geographical samples (n = 2,670) at 14 loci [30] (data source: http://www.pac.dfo-mpo.gc.ca/science/facilities-installations/pbs-sbp/mgl-lgm/data-donnees/index-eng.html). Hokkaido, Sea of Okhotsk coast (red circle); Hokkaido, Nemuro Strait (yellow circle); Hokkaido, eastern Pacific coast (brown circle); Hokkaido, western Pacific coast (dark green circle); Hokkaido, Sea of Japan coast (purple circle); Honshu, Pacific coast (light green circle); Honshu, Sea of Japan Coast (blue circle). S, September; O, October; N, November; D, December
In the Yurap River, our F ST value based on mtDNA between October and December (0.0529 [0.0158, 0.1072]) was 6.6 times larger than that between September and November based on msDNA (0.008 ± 0.002) [30]. Our estimate of the rate of global gene flow for msDNA (155.3 ± 4.7) was seven times larger than that of mtDNA (22.1 ± 6.9), suggesting that females return to natal rivers and spawning sites, but males stray into non-natal rivers to extend the chance of reproduction. Straying of males should serve to enhance genetic diversity in populations and adaptability to environmental changes. It is unclear if straying reflects a failure to return home or a decision to spawn elsewhere, but in either case, straying has been essential for the persistence and distribution of salmon [35]. Chinook salmon stray more (mean population range 0.11–34.6 %) than coho salmon (0.08–0.94 %), ocean-type Chinook (5.2–18.6 %) stray more than stream-type Chinook (0.11–10 %), and Chinook salmon (0.90–54.9 %) stray more than steelhead (0.30–2.3 %) [36].These patterns are largely the result of species-specific behavioral and endocrine factors during juvenile life stages [36]. The level of straying in hatchery chum salmon has not been assessed in Japan [2]. Further research is needed on chum salmon and pink salmon O. gorbuscha, which exhibit similar juvenile life histories (individuals typically migrate soon after emergence) [36]. The distributions of the mean pairwise F ST (0.0037 ± 0.0007) and the mean pairwise rate of gene flow (278 ± 49), estimated from msDNA allele frequencies of 26 samples (n = 2,670), suggested that straying is typical in chum salmon (Fig. 8). The global F ST values for masu salmon O. masou were 0.016–0.032 within the Uryu River system in Hokkaido [37], which were estimated from genotypes of 10 msDNA loci using the same software POPDIF. These point estimates were 2.3–4.6 times larger than our estimate of chum salmon (0.0069), which reflect the smaller rate of straying of masu salmon.
Distributions of a pairwise F ST and b the rate of gene flow (= the effective population size per generation) of chum salmon between rivers in Japan, inferred from microsatellite DNA allele frequencies in 26 geographical samples (n = 2,670) at 14 loci [19]
Genetic effects of hatchery release and roles of integrated hatchery management
Japanese populations had a similar number of haplotypes as in the Pacific Rim (Fig. 4a). However, the number of haplotypes ranged from two to seven in a population, and the loss of rare haplotypes was observed in all populations. Similar reductions in the numbers of haplotypes were observed in other foreign populations. In contrast, the number of microsatellite alleles was very high at 26.8 ± 11.3 (range 4.8–73.9) [30]. A large number of parents have been used since the mid-1970s when the stock enhancement program became effective. Currently, an annual total of 1.27 million fish (3.2 % of returned fish in 2012) are collected for hatchery broodstock from about 75 rivers in Hokkaido [2]. The large number of parent fish used for artificial fertilization may have contributed to maintaining the high number of microsatellite alleles (26.8 ± 11.3) [30]. However, it is unclear if long-term hatchery release is responsible for the loss of rare haplotypes, as the reproductive ecology of females might also be a factor.
Fish generally remain in a chum salmon hatchery (from eggs to release) for ~6 months. Survival rates in hatcheries are very high at >90 %. In contrast, survival rates should be very low in the wild, particularly during the early life stages. The rearing process in hatcheries should relax natural selection until release. In addition, eggs and sperm are taken artificially, so parent fish do not experience natural spawning. Carrying out this artificial fertilization process for more than 10 generations might reduce the natural spawning behavior performance of hatchery and wild fish. Such artificial selection could cause a reduction in the fitness of hatchery fish in the wild. Evidence for a reduction in reproductive success (RS) of hatchery fish has accumulated for Atlantic salmon [38, 39], steelhead trout [40–42], coho salmon [43], and Chinook salmon [44]. A Bayesian meta-analysis for the steelhead data showed that the average level of relative RS (RRS) implied the reproductive decline of hatchery fish, but RRS varies greatly depending on genetic cross, sex, year of release, and environmental conditions [45]. Reflecting the large variation in RRS in a super population, no RS difference between hatchery and wild fish has been found for steelhead trout [46], brown trout Salmo trutta [47], or coho salmon [48]. If the ratio of hatchery fish with lower RS is substantial in the stocked population, the total number of progeny recruited in the stocked population could be reduced in the next generation [49]. The carryover effect of reduced RS in the next generation, as estimated in a steelhead population [50], should amplify the reduction in recruitment.
The mechanisms responsible for reduced RS of hatchery fish are unknown [40]. The most likely cause is thought to be selection pressures associated with domestication in captivity [51]. Björnsson et al. [52] suggested that it is important not to lose sight of the fact that when handling expression data in endocrinology, it is the circulating hormones that stimulate target tissues. Gene expression mirrors the physiological activity of an organism. Highly significant differences in transcription levels have been found between farmed and wild Atlantic salmon [53]. A significant correlation between the domesticated/wild-type gene expression ratio and the transgenic/wild-type gene expression ratio for coho salmon was found [54]. Transgenesis of the growth hormone gene causes inferior reproductive behavior and reduces fertilization success of Atlantic salmon [55]. These findings provide an important suggestion that enhanced growth in captivity causes a reduction in reproductive performance of hatchery fish. Actually, hatchery-reared steelhead are almost ubiquitously released as yearlings, whereas wild steelhead typically spend two or more years in freshwater before migrating to sea [56]. It is crucial to discover the essential genetic cascades related to survival and reproductive traits, and to identify the main trigger genes, which will determine the mechanisms of the fitness reduction caused by captive rearing [57]. The results will contribute to the development of juvenile production techniques that help to conserve natural populations.
The duration of hatchery rearing for chum salmon (~6 months) is about half that for steelhead. The growth of hatchery-reared chum salmon is also similar to wild fish and is not enhanced in the hatchery, as in steelhead and farmed Atlantic salmon. The effect of domestication might be smaller in chum salmon than in other salmon, except in pink salmon. No significant difference in RS between hatchery and wild chum salmon has been found [58]. The RS of wild and hatchery chum salmon has not been evaluated in Japan, so it is unknown if hatchery fish have lower RS in the wild and if releases reduce the RS of the population. It should be noted that DNA parentage assignment for the precise estimation of RS requires a complete sampling of the parent and progenies for hatchery and wild fish [59]. The number of returns and return rates in the Kitami region have continued to increase in the last two decades (Fig. 1b), showing that reductions in the survival rate of hatchery-released fish are unlikely. However, a consistent decreasing trend in the number of returns and the return rate since about 2005 in other regions in Hokkaido clearly indicates a survival decline of hatchery-released fish. Mizuno et al. [60] suggested the need for improving juvenile quality in the areas where recent returns have been decreasing in order to recover the chum salmon stocks.
Efforts to enhance the early-run population have been encouraged since the early 1980s, and resulted in a change in run timing [2], as found in coho salmon [48]. Miyakoshi et al. [2] demonstrated the long-term change (1970–2010) in the distribution of adult chum salmon captured in enhanced rivers in Hokkaido. There were both early- and late-run populations of chum salmon in Hokkaido until the early 1980s, and the numbers of fish in the two runs were almost the same (see Fig. 8 in Miyakoshi et al. [2]). The late-run population had almost disappeared by the late 1990s. The late-run population has continued to decrease because adults captured late in the season for several years in the 1980s were not used for hatchery fertilization, but instead were simply sold. Hatchery managers have recently become aware that such extreme intentional selection is undesirable and have made an effort to escape the late-run fish, as mentioned above. The late-run population has been recovering gradually, suggesting the effectiveness of such escapement in enhanced rivers (Fig. 8 in Miyakoshi et al. [2]). The run-timing distribution for 1970–1975 should reflect that of the natural (original) populations of chum salmon in Hokkaido, because that period was the beginning of the increase in returned fish due to success of the hatchery program. The very small population differentiation of chum salmon was evaluated by neutral genetic markers. The F ST analysis evaluates differences in allele or haplotype frequencies and might not reflect the genetic characteristics of the indigenous demes, but the present run-timing distribution suggests that indigenous individuals remain in many rivers even with hatcheries. Therefore, escapement in enhanced and non-enhanced rivers throughout the run season should enable the recovery of the natural (original) genetic component. Further efforts for the escapement of both early- and late-run fish are key to the recovery and maintenance of natural populations of chum salmon.
The escapement of hatchery [H] fish into the wild [W] population allows for the reproduction between genetic crosses of [HH], [HW], and [WW], which creates three types of wild fish, denoted as W [HH], W [HW], and W [WW]. There are also three types of hatchery fish, such as H [HH], H [HW] and H [WW]. Substantial introgression of hatchery-released fish into a wild population has been observed, even after several generations [17]. Considering long-term intensive hatchery release, almost all chum salmon returning to the Kitami region may be hatchery descendants. The increasing trend in return rate may suggest that hatchery-wild integrated reproduction acts to compensate for domestication selection caused by captive rearing and artificial fertilization in hatcheries. The Sea of Okhotsk region is considered as a genetic management unit, and the genetic difference between hatchery (early-run) and wild fish (late-run) is very small (F ST = 0.008–0.010) [30]. This coincides with the concept of the genetically-integrated hatchery program [10]. In the successful salmon stock enhancement in southeast Alaska, hatcheries are located away from significant wild stocks [61], regarded as a genetically-segregated hatchery program [10]. However, the contribution of hatchery-origin chum salmon is 78 % in southeast Alaska [61], showing that any hatchery program is an integrated one within enhanced regions. Under integrated management, the idea of zoning management for enhanced and non-enhanced rivers in Hokkaido [13, 14] is useful both for conservation and for the production of chum salmon. Spawning habitats suitable for the reproduction of chum salmon are estimated to comprise 891 km in the Kitami region, including the Shiretoko area (Urabe H, SFFRI, unpublished data). It is crucial to allow escapements throughout the run season in order to sustain the habitat capacity of enhanced and non-enhanced rivers. Establishing river-specific escapement goals in enhanced rivers is needed. Marking and genetic monitoring will contribute to an evaluation of the effectiveness of the integrated hatchery program. Furthermore, the restoration of rivers, including dams [13, 14], increases spawning habitat capacity.
Concluding remarks
The number of returns and return rates of hatchery fish have continued to increase in the past two decades in the Sea of Okhotsk region, supported by the favorable ocean and climate conditions. The increasing trend in return rates suggests that a survival reduction of hatchery-released fish in the Kitami chum salmon population does not occur. On the other hand, a consistent decreasing trend in the number of returns and the return rate since about 2005 in other regions in Hokkaido clearly indicates a survival decline of hatchery-released fish. However, it is unknown if hatchery fish have lower RS in the wild and whether releases reduce the RS of the stocked population. It is crucial to determine the mechanisms of the fitness reduction caused by captive rearing. New approaches are needed to discover the essential genetic cascades related to the survival and reproductive traits and to identify the main trigger genes, which will contribute to developing juvenile production techniques aimed at conserving natural populations.
The genetic diversity of Japanese chum salmon is higher or similar compared with other populations in the Pacific Rim. However, the number of haplotypes ranged from two to seven in a population, and a loss of rare haplotypes was observed in all populations. In contrast, the number of microsatellite alleles was very high at 26.8 ± 11.3 (range 4.8–73.9). A tentative estimate of N e /N ratio for the Kitami hatchery program was >0.15 % under the present high fishing pressure. The integrated hatchery-wild reproduction system, realized by a large number of broodstock in hatcheries and escapement into enhanced and non-enhanced rivers, maintains the present genetic diversity. However, it is unclear if long-term hatchery release is responsible for the loss of rare haplotypes, which might be caused by the reproductive ecology of females. Six regional populations (four on Hokkaido and two on Honshu) are assumed to occur within Japan, which suggests the management units of Japanese chum salmon. However, genetic differentiation is very low, and the population structure is not rigid. The nested population structure in Hokkaido might reflect the transplantation history of hatchery fish. The large gene flow suggests that straying, particularly by males, is a chum salmon strategy to maintain genetic diversity. Early and late-run populations are not genetically distinct in terms of F ST.
Intensive hatchery enhancement for the early run populations has changed the distribution of run timing for the chum salmon populations in Hokkaido. Recent escapement practices of late-run fish have gradually recovered the late-run populations. Hatchery releases serve to maintain the genetic composition of the early run, whereas escapement practices recover the late run, which contributes to maintaining the genetic characteristics of the natural (original) populations. Further efforts for escapement of both early- and late-run fish are the key to recovering and maintaining natural populations of chum salmon. Surplus spawning habitat capacity exists in the Kitami region including the Shiretoko area. The challenge is to extend present escapement practices to other enhanced rivers with allowable escapements for habitat capacity. The restoration of rivers and dams increases spawning habitat capacity. A region-wide integrated hatchery management plan with river-specific escapement goals in enhanced rivers will recover the natural populations and contribute to sustainable chum salmon fisheries in the future. New research is needed to evaluate the effectiveness of the integrated hatchery program, which will contribute to the minimization of ecological and genetic risks for hatchery fish.
References
Kaeriyama M (1999) Hatchery programmes and stock management of salmonid populations in Japan. In: Howell BR, Moksness E, Svåsand T (eds) Stock enhancement and sea ranching. Blackwell, Oxford, pp 153–167
Miyakoshi Y, Nagata M, Kitada S, Kaeriyama M (2013) Historical and current hatchery programs and management of chum salmon in Hokkaido, northern Japan. Rev Fish Sci 21:469–479
Beamish RJ, Bouillon DR (1993) Pacific salmon production trends in relation to climate. Can J Fish Aquat Sci 50:1002–1016
Kaeriyama M, Seo H, Kudo H (2009) Trends in run size and carrying capacity of Pacific salmon in the North Pacific Ocean. N Pac Anadr Fish Comm Bull 5:293–302
Morita K, Saito T, Miyakoshi Y, Fukuwaka M, Nagasawa T, Kaeriyama M (2006) A review of Pacific salmon hatchery programmes on Hokkaido Island, Japan. ICES J Mar Sci 63:1353–1363
Miyakoshi Y, Urave H, Saneyoshi H, Aoyama T, Sakamoto H, Ando D, Kasugai K, Mishima Y, Takada M, Nagata M (2012) The occurrence and run timing of naturally spawning chum in northern Japan. Environ Biol Fish 94:197–206
Hilborn R (1992) Hatcheries and the future of salmon in the northwest. Fisheries 17:5–8
Waples RS (1999) Dispelling some myths about hatcheries. Fisheries 24:12–21
Brannon EL, Amend DF, Cronin MA, Lannan JE, LaPatra S, McNeil WJ, Noble RE, Smith CE, Talbot AJ, Wedemeyer GA, Westers H (2004) The controversy about salmon hatcheries. Fisheries 29:12–31
Mobrand L, Barr J, Blankenship HL, Campton DE, Evelyn TTP, Flagg TA, Mahnken CVW, Seeb LW, Seidel RR, Smoker WW (2005) Hatchery reform in Washington state: principles and emerging issues. Fisheries 30:11–23
Waples RS, Drake J (2004) Risk/benefit considerations for marine stock enhancement: a Pacific salmon perspective. In: Leber KM, Kitada S, Svåsand T, Blankenship HL (eds) Stock enhancement and sea ranching, 2nd edn. Blackwell, Oxford, pp 260–306
Kaeriyama M, Edpalina RR (2004) Evaluation of the biological interaction between wild and hatchery population for sustainable fisheries management of Pacific salmon. In: Leber KM, Kitada S, Svåsand T, Blankenship HL (eds) Stock enhancement and sea ranching, 2nd edn. Blackwell, Oxford, pp 247–259
Kaeriyama M, Seo H, Kudo H, Nagata M (2012) Perspectives on wild and hatchery salmon interactions at sea, potential climate effects of Japanese chum salmon at the need for sustainable salmon fisheries management reform in Japan. Environ Biol Fish 94:165–177
Nagata M, Miyakoshi Y, Urabe H, Fujiwara M, Sasaki Y, Kasugai K, Torao M, Ando D, Kaeriyama M (2012) An overview of salmon enhancement and the need to manage and monitor natural spawning in Hokkaido, Japan. Environ Biol Fish 94:311–323
Kitada S, Kishino H (2006) Lessons learned from Japanese marine finfish stock enhancement programmes. Fish Res 80:101–112
Kitada S, Shishidou H, Sugaya T, Kitakado T, Hamasaki K, Kishino H (2009) Genetic effects of the long-term stock enhancement programs. Aquaculture 290:69–79
Nakajima K, Kitada S, Habara Y, Sano S, Yokoyama E, Sugaya T, Iwamoto A, Kishino H, Hamasaki K (2014) Genetic effects of marine stock enhancement: a case study based on the highly piscivorous Japanese Spanish mackerel. Can J Fish Aquat Sci. doi:10.1139/cjfas-2013-0418.21
Morita K, Takahashi S, Ohkuma K, Nagasawa T (2013) Estimation of the proportion of wild chum salmon Oncorhynchus keta in Japanese hatchery rivers (in Japanese with English abstract). Nippon Suisan Gakkaishi 79:206–213
Frankham R (1995) Effective population size/adult population size ratios in wildlife: a review. Genet Res 66:95–107
Hedgecock D (1994) Does variance in reproductive success limit effective population sizes of marine organisms? In: Beaumont AR (ed) Genetics and evolution of aquatic organisms. Chapman and Hall, London, pp 122–134
Grant WS, Waples RS (2000) Spatial and temporal scales of genetic variability in marine and anadromous species: implications for fisheries oceanography. In: Harrison PJ, Parsons TR (eds) Fisheries oceanography. Blackwell Science, Cambridge, pp 61–93
Hutchinson WF, van Oosterhout C, Rogers SI, Carvalho GR (2003) Temporal analysis of archived samples indicates marked genetic changes in declining North Sea cod (Gadus morhua). Proc Royal Soc B-Biol Sci 270:2125–2132
Hauser L, Adcock GJ, Smith PJ, Bernal Ramirez JH, Carvalho GR (2002) Loss of microsatellite diversity and low effective population size in an overexploited population of New Zealand snapper (Pagrus auratus). Proc Natl Acad Sci USA 99:11742–11747
Turner TF, Wares JP, Gold JR (2002) Genetic effective size is three orders of magnitude smaller than adult census size in an abundant, estuarine-dependant marine fish (Sciaenops ocellatus). Genetics 162:1329–1339
Cushing DH (1995) Population production and regulation in the sea: a fisheries perspective. Cambridge University Press, Cambridge
Bartley D, Bagley M, Gall G, Bently B (1992) Use of linkage disequilibrium data to estimate effective size of hatchery and natural fish populations. Cons Biol 6:365–375
Allendorf FW, Bayles D, Bottom D, Currens KP, Frissell CA, Hankin D, Lichatowich JA, Nehlsen W, Trotter PC, Williams TH (1997) Prioritizing Pacific salmon stocks for conservation. Cons Biol 11:140–152
Schwartz MK, Luikart G, Waples RS (2006) Genetic monitoring as a promising tool for conservation and management. Trends Ecol Evol 22:25–33
Sato S, Kojima H, Ando J, Ando H, Wilmot RL, Seeb LW, Efremov V, LeClair L, Buchholz W, Jin DH, Urawa S, Kaeriyama M, Urano A, Abe S (2004) Genetic population structure of chum salmon in the Pacific Rim inferred from mitochondrial DNA sequence variation. Environ Biol Fish 69:37–50
Beacham TD, Sato S, Urawa S, Lei KD, Wetklo M (2008) Population structure and stock identification of chum salmon Oncorhynchus keta from Japan determined by microsatellite DNA variation. Fish Sci 74:983–994
Seeb LW, Templin WD, Sato S, Abe S, Warheit K, Park JY, Seeb JE (2011) Single nucleotide polymorphisms across a species’ range: implications for conservation studies of Pacific salmon. Molec Ecol Res 11(Suppl 1):195–217
Yokotani R, Azuma N, Kudo H, Abe S, Kaeriyama M (2009) Genetic differentiation between early- and late-run populations of chum salmon (Oncorhynchus keta) naturally spawned in the Yurappu River inferred from mitochondrial DNA analysis. Fish Genet Breed Sci 39:1–8
Sato S, Ando J, Ando H, Urawa S, Urano A, Abe S (2001) Genetic variation among Japanese populations of chum salmon inferred from the nucleotide sequences of the mitochondrial DNA control region. Zool Sci 18:99–106
Kitada S, Kitakado T, Kishino H (2007) Empirical Bayes inference of F ST and its distribution in the genome. Genetics 177:861–873
Quinn TP (2005) The behavior and ecology of Pacific salmon and trout. University of Washington Press, Seattle
Westley PAH, Quinn TP, Dittman AH (2013) Rates of straying by hatchery-produced Pacific salmon (Oncorhynchus spp.) and steelhead (Oncorhynchus mykiss) differ among species, life history types, and populations. Can J Fish Aquat Sci 70:735–746
Kitanishi S, Yamamoto Y, Edo K, Higashi S (2012) Influences of habitat fragmentation by damming on the genetic structure of masu salmon populations in Hokkaido, Japan. Conserv Genet 13:1017–1026
Fleming I, Hindar K, Mjølnerød IB, Jonsson B, Balstad T, Lamberg A (2000) Lifetime success and interactions of farm salmon invading a native population. Proc R Soc Lond B 267:1517–1523
McGinnity P, Prodöhl P, Ferguson A, Hynes R, Ó Maoiléidigh N, Baker N, Cotter D, O’Hea B, Cooke D, Rogan G, Taggart J, Cross T (2003) Fitness reduction and potential extinction of wild populations of Atlantic salmon Salmo salar as a result of interactions with escaped farm salmon. Proc R Soc Lond B 270:2443–2450
Araki H, Cooper B, Blouin MS (2007) Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science 318:100–103
Berntson EA, Carmichael RW, Flesher MW, Ward EJ, Moran P (2011) Diminished reproductive success of steelhead from a hatchery supplementation program (Little Sheep Creek, Imnaha Basin, Oregon). Trans Am Fish Soc 140:685–698
Christie MR, Marine ML, French RA, Blouin MS (2012) Genetic adaptation to captivity can occur in a single generation. PNAS 109:238–242
Thérault V, Moyer G, Jackson LS, Blouin MS, Banks MA (2011) Reduced reproductive success of hatchery coho salmon in the wild: insights into most likely mechanisms. Molec Ecol 20:1860–1869
Anderson JH, Faulds PL, Atlas WI, Quinn TP (2013) Reproductive success of captively bred and naturally spawned Chinook salmon colonizing newly accessible habitat. Evol Appl 6:165–179
Kitada S, Kishino H, Hamasaki K (2011) Bias and significance of relative reproductive success estimates based on steelhead trout (Oncorhynchus mykiss) data: a Bayesian metaanalysis. Can J Fish Aquat Sci 68:1827–1835
Araki H, Ardren WR, Olsen E, Cooper B, Blouin MS (2007) Reproductive success of captive-bred steelhead trout in the wild: evaluation of three hatchery programs in the Hood River. Conserv Biol 21:181–190
Dannewitz J, Petersson E, Dahl J, Prestegaard T, Löf AC, Järvi T (2004) Reproductive success of hatchery-produced and wild-born brown trout in an experimental stream. J Appl Ecol 41(2):355–364
Ford MJ, Fuss H, Boelts B, LaHood E, Hard J, Miller J (2006) Changes in run timing and natural smolt production in a naturally spawning coho salmon (Oncorhynchus kisutch) population after 60 years of intensive hatchery supplementation. Can J Fish Aquat Sci 63:2343–2355
Chilcote MW, Goodson KW, Falcy MR (2011) Reduced recruitment performance in natural populations of anadromous salmonids associated with hatchery-reared fish. Can J Fish Aquat Sci 68:511–522
Araki H, Cooper B, Blouin MS (2009) Carry-over effect of captive breeding reduces reproductive fitness of wild-born descendants in the wild. Biol Lett 5:621–624
Araki H, Berejikian BA, Ford MJ, Blouin MS (2008) Fitness of hatchery-reared salmonids in the wild. Evol Appl 1:342–355
Björnsson BT, Stefansson SO, McCormick SD (2011) Environmental endocrinology of salmon smoltification. Gen Comp Endocrinol 170:290–298
Roberge C, Normandeau E, Einum S, Guderley H, Bernatchez L (2008) Genetic consequences of interbreeding between farmed and wild Atlantic salmon: insights from the transcriptome. Mol Ecol 17:314–324
Devlin RH, Sakhrani D, Tymchuk WE, Rise ML, Goh B (2009) Domestication and growth hormone transgenesis cause similar changes in gene expression in coho salmon (Oncorhynchus kisutch). Proc Natl Acad Sci USA 106(9):3047–3052
Moreau DTR, Conway C, Fleming IA (2011) Reproductive performance of alternative male phenotypes of growth hormone transgenic Atlantic salmon (Salmo salar). Evol Appl 4:736–748
Berejikian BA, Larsen DA, Swanson P, Moore ME, Tatara CP, Gale WL, Pasley CR, Beckman BR (2012) Development of natural growth regimes for hatchery-reared steelhead to reduce residualism, fitness loss, and negative ecological interactions. Environ Biol Fish 94:29–44
Nakamichi R, Kishino H, Kitada S (2013) A novel method to identify key factors of the gene regulatory network behind salmonids reproductive behavior using directed graphical modeling. N Pac Anadr Fish Comm Tec Rep 9:50–53
Berejikian BA, Van Doornik DM, Scheurer JA, Bush R (2009) Reproductive behavior and relative reproductive success of natural- and hatchery-origin Hood Canal summer chum salmon (Oncorhynchus keta). Can J Fish Aquat Sci 66:781–789
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Molec Ecol 7:639–655
Mizuno S, Nakajima M, Naito K, Koyama T, Saneyoshi H, Kobayashi M, Koide N, Ueda H (2010) Physiological impacts of high rearing density on chum salmon Oncorhynchus keta fry. Aquaculture Sci 58:387–399
Heard WR (2012) Overview of salmon stock enhancement in southeast Alaska and compatibility with maintenance of hatchery and wild stocks. Environ Biol Fish 94:273–283
Acknowledgments
I am indebted to Y. Miyakoshi (SFFRI), who provided data for Figs. 1 and 3 with valuable information. I thank M. Kaeriyama (Hokkaido University) and M. Nagata (SFFRI) for constructive comments on the earlier version of the manuscript. I also thank H. Urabe and Y. Sasaki (SFFRI), K. Fukuda (HFFCA), N. Masukawa, and O. Ishizuka (KSEPA) for providing useful materials. This study was supported by a JSPS Grant-in-Aid for Scientific Research (B) 25280006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kitada, S. Japanese chum salmon stock enhancement: current perspective and future challenges. Fish Sci 80, 237–249 (2014). https://doi.org/10.1007/s12562-013-0692-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-013-0692-8