Abstract
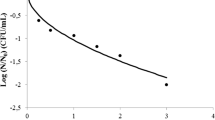
High pressure inactivation of hepatitis A virus (HAV) within oysters bioaccumulated under simulated natural conditions to levels >105 PFU/oyster has been evaluated. Five minute treatments at 20°C were administered at 350, 375, and 400 MegaPascals (MPa). Shucked and whole-in-shell oysters were directly compared to determine if there were any differences in inactivation levels. For whole-in-shell oysters and shucked oysters, average values obtained were 2.56 and 2.96 log10 inactivation of HAV, respectively, after a 400-MPa treatment. Results indicate that there is no significant inactivation difference (P = 0.05) between inactivation for whole-in-shell oysters as compared to shucked oysters observed for all pressure treatments. This study indicates that commercial high pressure processing applied to whole-in-shell oysters will be capable of inactivating HAV pathogens.

Similar content being viewed by others
References
Berlin, D. L., Herson, D. S., Hicks, D. T., & Hoover, D. G. (1999). Response of pathogenic Vibrio species to high hydrostatic pressure. Applied and Environmental Microbiology, 65, 2776–2780.
Calci, K. R., Meade, G. K., Tetzloff, R. C., & Kingsley, D. H. (2005). High-pressure inactivation of hepatitis A virus within oysters. Applied and Environmental Microbiology, 71, 339–343.
Chen, H., Hoover, D. G., & Kingsley, D. H. (2005). Temperature and treatment time influence high hydrostatic pressure inactivation of feline calicivirus, a norovirus surrogate. Journal of Food Protection, 68, 2389–2394.
Cook, D. W. (2003). Sensitivity of Vibrio species in phosphate buffer saline and in oysters to high pressure processing. Journal of Food Protection, 66, 2276–8266.
Kingsley, D. H., & Chen, H. (2009). Influence of pH, salt, and temperature on pressure inactivation of hepatitis A virus. International Journal of Food Microbiology, 130, 61–64.
Kingsley, D. H., Guan, D., Hoover, D. G., & Chen, H. (2006). Inactivation of hepatitis A virus by high pressure processing: the role of temperature and pressure oscillation. Journal of Food Protection, 69, 2454–2459.
Kingsley, D. H., Holliman, D. R., Calci, K. R., Chen, H., & Flick, G. J. (2007). Inactivation of a norovirus by high pressure processing. Applied and Environmental Microbiology, 73, 581–585.
Kingsley, D. H., Hoover, D., Papafragkou, E., & Richards, G. P. (2002). Inactivation of hepatitis A virus and a calicivirus by high hydrostatic pressure. Journal of Food Protection, 65, 1605–1609.
Kingsley, D. H., & Richards, G. P. (2003). Persistence of hepatitis A virus within oysters. Journal of Food Protection, 66, 331–334.
Kural, A. G., Shearer, A. E. H., Kingsley, D. H., & Chen, H. (2008). Pressure inactivation of Vibrio parahaemolyticus in oysters—the influence of pressure level and treatment temperature. International Journal of Food Microbiology, 127, 1–5.
Loisy, F., Atmar, R. L., LeSaux, J. C., Cohen, J., Caprais, M. P., Pommepuy, M., et al. (2005). Use of rotavirus virus like particles as surrogates to evaluate virus persistence in shellfish. Applied and Environmental Microbiology, 71, 6049–6053.
Oliver, J. D., & Kaper, J. (2001). Vibrio species. In M. P. Doyle, et al. (Eds.), Food microbiology: fundamentals and frontiers (pp. 263–300). Washington, DC: ASM Press.
Richards, G. P., & Watson, M. A. (2001). Immunochemiluminescent focus assays for the quantitation of hepatitis A virus and rotavirus in cell cultures. Journal of Virological Methods, 94, 69–80.
Straub, T. M., Höner zu Bentrup, K., Orosz-Coghlan, P., Dohnalkova, A., Mayer, B. K., Bartholomew, R. A., et al. (2007). In vitro cell culture infectivity assay for human noroviruses. Emerging Infectious Diseases, 13, 396–403.
Wobus, C. E., Thackray, L. B., & Virgin, H. B., IV (2006). Murine norovirus: a model system to study norovirus biology and pathogenesis. Journal of Virology, 80, 5104–5112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Rights and permissions
About this article
Cite this article
Kingsley, D.H., Calci, K., Holliman, S. et al. High Pressure Inactivation of HAV Within Oysters: Comparison of Shucked Oysters with Whole-In-Shell Meats. Food Environ Virol 1, 137–140 (2009). https://doi.org/10.1007/s12560-009-9018-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-009-9018-5