Abstract
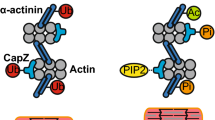
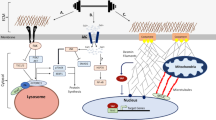
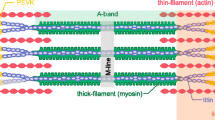
All cells sense force and build their cytoskeleton to optimize function. How is this achieved? Two major systems are involved. The first is that load deforms specific protein structures in a proportional and orientation-dependent manner. The second is post-translational modification of proteins as a consequence of signaling pathway activation. These two processes work together in a complex way so that local subcellular assembly as well as overall cell function are controlled. This review discusses many cell types but focuses on striated muscle. Detailed information is provided on how load deforms the structure of proteins in the focal adhesions and filaments, using α-actinin, vinculin, talin, focal adhesion kinase, LIM domain-containing proteins, filamin, myosin, titin, and telethonin as examples. Second messenger signals arising from external triggers are distributed throughout the cell causing post-translational or chemical modifications of protein structures, with the actin capping protein CapZ and troponin as examples. There are numerous unanswered questions of how mechanical and chemical signals are integrated by muscle proteins to regulate sarcomere structure and function yet to be studied. Therefore, more research is needed to see how external triggers are integrated with local tension generated within the cell. Nonetheless, maintenance of tension in the sarcomere is the essential and dominant mechanism, leading to the well-known phrase in exercise physiology: “use it or lose it.”




Similar content being viewed by others
References
Ait-Mou Y, Hsu K, Farman GP, Kumar M, Greaser ML, Irving TC, de Tombe PP (2016) Titin strain contributes to the Frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins. Proc Natl Acad Sci U S A 113:2306–2311. https://doi.org/10.1073/pnas.1516732113
Altman D, Sweeney HL, Spudich JA (2004) The mechanism of myosin VI translocation and its load-induced anchoring. Cell 116:737–749. https://doi.org/10.1016/s0092-8674(04)00211-9
Anthis NJ et al (2009) The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J 28:3623–3632. https://doi.org/10.1038/emboj.2009.287
Arold ST, Hoellerer MK, Noble ME (2002) The structural basis of localization and signaling by the focal adhesion targeting domain. Structure (London, England : 1993) 10:319–327. https://doi.org/10.1016/s0969-2126(02)00717-7
Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL (2004) Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol 286:C355–C364. https://doi.org/10.1152/ajpcell.00211.2003
Bauer MS et al (2019) Structural and mechanistic insights into mechanoactivation of focal adhesion kinase. Proc Natl Acad Sci U S A 116:6766–6774. https://doi.org/10.1073/pnas.1820567116
Bays JL, DeMali KA (2017) Vinculin in cell-cell and cell-matrix adhesions. Cell Mol Life Sci CMLS 74:2999–3009. https://doi.org/10.1007/s00018-017-2511-3
Bertz M, Wilmanns M, Rief M (2009) The titin-telethonin complex is a directed, superstable molecular bond in the muscle Z-disk. Proc Natl Acad Sci U S A 106:13307–133310. https://doi.org/10.1073/pnas.0902312106
Burgoyne T et al (2019) Three-dimensional structure of the basketweave Z-band in midshipman fish sonic muscle. Proc Natl Acad Sci 116:15534–15539. https://doi.org/10.1073/pnas.1902235116
Buyandelger B, Ng KE, Miocic S, Piotrowska I, Gunkel S, Ku CH, Knöll R (2011) MLP (muscle LIM protein) as a stress sensor in the heart. Pflugers Arch - Eur J Physiol 462:135–142. https://doi.org/10.1007/s00424-011-0961-2
Byrne BJ, Kaczorowski YJ, Coutu MD, Craig SW (1992) Chicken vinculin and meta-vinculin are derived from a single gene by alternative splicing of a 207-base pair exon unique to meta-vinculin. J Biol Chem 267:12845–12850. https://doi.org/10.1016/S0021-9258(18)42353-8
Byron KL, Puglisi JL, Holda JR, Eble D, Samarel AM (1996) Myosin heavy chain turnover in cultured neonatal rat heart cells: effects of [Ca2+]i and contractile activity. Am J Phys 271:C01447–C01456. https://doi.org/10.1152/ajpcell.1996.271.5.C01447
Caldwell JE, Heiss SG, Mermall V, Cooper JA (1989) Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry 28:8506–8514. https://doi.org/10.1021/bi00447a036
Candasamy AJ et al (2014) Phosphoregulation of the titin-cap protein telethonin in cardiac myocytes. J Biol Chem 289:1282–1293. https://doi.org/10.1074/jbc.M113.479030
Capitanio M et al (2012) Ultrafast force-clamp spectroscopy of single molecules reveals load dependence of myosin working stroke. Nat Methods 9:1013–1019. https://doi.org/10.1038/nmeth.2152
Cazorla O, Wu Y, Irving TC, Granzier H (2001) Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ Res 88:1028–1035. https://doi.org/10.1161/hh1001.090876
Ceccarelli DF, Song HK, Poy F, Schaller MD, Eck MJ (2006) Crystal structure of the FERM domain of focal adhesion kinase. J Biol Chem 281:252–259. https://doi.org/10.1074/jbc.M509188200
Chinthalapudi K, Rangarajan ES, Brown DT, Izard T (2016) Differential lipid binding of vinculin isoforms promotes quasi-equivalent dimerization. Proc Natl Acad Sci U S A 113:9539–9544. https://doi.org/10.1073/pnas.1600702113
Chopra A et al (2018) Force generation via β-cardiac Myosin, Titin, and α-Actinin drives cardiac sarcomere assembly from cell-matrix adhesions. Dev Cell 44:87–96.e85. https://doi.org/10.1016/j.devcel.2017.12.012
Chu PH, Bardwell WM, Gu Y, Ross J Jr, Chen J (2000) FHL2 (SLIM3) is not essential for cardiac development and function. Mol Cell Biol 20:7460–7462. https://doi.org/10.1128/mcb.20.20.7460-7462.2000
Chu M et al (2011) Serine-910 phosphorylation of focal adhesion kinase is critical for sarcomere reorganization in cardiomyocyte hypertrophy. Cardiovasc Res 92:409–419. https://doi.org/10.1093/cvr/cvr247
Danieli A, Martens S (2018) p62-mediated phase separation at the intersection of the ubiquitin-proteasome system and autophagy. J Cell Sci 131. https://doi.org/10.1242/jcs.214304
Davis J et al (2016) A tension-based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell 165:1147–1159. https://doi.org/10.1016/j.cell.2016.04.002
de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC (2010) Myofilament length dependent activation. J Mol Cell Cardiol 48:851–858. https://doi.org/10.1016/j.yjmcc.2009.12.017
Dedden D, Schumacher S, Kelley CF, Zacharias M, Biertümpfel C, Fässler R, Mizuno N (2019) The architecture of Talin1 reveals an autoinhibition mechanism. Cell 179:120–131.e113. https://doi.org/10.1016/j.cell.2019.08.034
del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP (2009) Stretching single talin rod molecules activates vinculin binding. Science 323:638–641. https://doi.org/10.1126/science.1162912
Di Paolo G et al (2002) Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature 420:85–89. https://doi.org/10.1038/nature01147
Dix DJ, Eisenberg BR (1991a) Distribution of myosin mRNA during development and regeneration of skeletal muscle fibers. Dev Biol 143:422–426. https://doi.org/10.1016/0012-1606(91)90093-i
Dix DJ, Eisenberg BR (1991b) Redistribution of myosin heavy chain mRNA in the midregion of stretched muscle fibers. Cell Tissue Res 263:61–69. https://doi.org/10.1007/bf00318400
Dupont S et al (2011) Role of YAP/TAZ in mechanotransduction. Nature 474:179–183. https://doi.org/10.1038/nature10137
Edwards M, Zwolak A, Schafer DA, Sept D, Dominguez R, Cooper JA (2014) Capping protein regulators fine-tune actin assembly dynamics. Nat Rev Mol Cell Biol 15:677–689. https://doi.org/10.1038/nrm3869
Eisenberg B (1983) Section 10. Quantitative ultrastructure of mammalian skeletal muscle. Handbook of Physiology Peachey LD and Adrian RH, editors Bethesda, MD: American Physiological Society 73112. https://doi.org/10.1002/cphy.cp100103
Eisenberg BR, Goldspink PH, Wenderoth MP (1991) Distribution of myosin heavy chain mRNA in normal and hyperthyroid heart. J Mol Cell Cardiol 23:287–296. https://doi.org/10.1016/0022-2828(91)90065-t
Farman GP, Gore D, Allen E, Schoenfelt K, Irving TC, de Tombe PP (2011) Myosin head orientation: a structural determinant for the Frank-Starling relationship. Am J Phys Heart Circ Phys 300:H2155–H2160. https://doi.org/10.1152/ajpheart.01221.2010
Forkey JN, Quinlan ME, Shaw MA, Corrie JE, Goldman YE (2003) Three-dimensional structural dynamics of myosin V by single-molecule fluorescence polarization. Nature 422:399–404. https://doi.org/10.1038/nature01529
Franchini KG, Torsoni AS, Soares PH, Saad MJ (2000) Early activation of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overload in the rat heart. Circ Res 87:558–565. https://doi.org/10.1161/01.res.87.7.558
Frank D, Kuhn C, Katus HA, Frey N (2006) The sarcomeric Z-disc: a nodal point in signalling and disease. Journal of Molecular Medicine (Berlin, Germany) 84:446–468. https://doi.org/10.1007/s00109-005-0033-1
Friedrich FW et al (2014) FHL2 expression and variants in hypertrophic cardiomyopathy. Basic Res Cardiol 109:451. https://doi.org/10.1007/s00395-014-0451-8
Gao QQ, McNally EM (2015) The dystrophin complex: structure, function, and implications for therapy. Compr Physiol 5:1223–1239. https://doi.org/10.1002/cphy.c140048
Gautel M, Djinović-Carugo K (2016) The sarcomeric cytoskeleton: from molecules to motion. J Exp Biol 219:135–145. https://doi.org/10.1242/jeb.124941
Genini M, Schwalbe P, Scholl FA, Remppis A, Mattei MG, Schäfer BW (1997) Subtractive cloning and characterization of DRAL, a novel LIM-domain protein down-regulated in rhabdomyosarcoma. DNA Cell Biol 16:433–442. https://doi.org/10.1089/dna.1997.16.433
Gingras AR, Ziegler WH, Frank R, Barsukov IL, Roberts GC, Critchley DR, Emsley J (2005) Mapping and consensus sequence identification for multiple vinculin binding sites within the talin rod. J Biol Chem 280:37217–37224. https://doi.org/10.1074/jbc.M508060200
Goldstein MA, Michael LH, Schroeter JP, Sass RL (1988) Structural states in the Z band of skeletal muscle correlate with states of active and passive tension. J Gen Physiol 92:113–119. https://doi.org/10.1085/jgp.92.1.113
Goldstein MA, Michael LH, Schroeter JP, Sass RL (1989) Two structural states of Z-bands in cardiac muscle. Am J Phys 256:H552–H559. https://doi.org/10.1152/ajpheart.1989.256.2.H552
Grabarek Z (2011) Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins. Biochim Biophys Acta 1813:913–921. https://doi.org/10.1016/j.bbamcr.2011.01.017
Graham ZA, Gallagher PM, Cardozo CP (2015) Focal adhesion kinase and its role in skeletal muscle. J Muscle Res Cell Motil 36:305–315. https://doi.org/10.1007/s10974-015-9415-3
Gräter F, Shen J, Jiang H, Gautel M, Grubmüller H (2005) Mechanically induced titin kinase activation studied by force-probe molecular dynamics simulations. Biophys J 88:790–804. https://doi.org/10.1529/biophysj.104.052423
Greenberg MJ, Shuman H, Ostap EM (2014) Inherent force-dependent properties of β-cardiac myosin contribute to the force-velocity relationship of cardiac muscle. Biophys J 107:L41–l44. https://doi.org/10.1016/j.bpj.2014.11.005
Hanson J, Huxley HE (1953) Structural basis of the cross-striations in muscle. Nature 172:530–532. https://doi.org/10.1038/172530b0
Hartman TJ, Martin JL, Solaro RJ, Samarel AM, Russell B (2009) CapZ dynamics are altered by endothelin-1 and phenylephrine via PIP2- and PKC-dependent mechanisms. Am J Physiol Cell Physiol 296:C1034–C1039. https://doi.org/10.1152/ajpcell.00544.2008
Hayashi I, Vuori K, Liddington RC (2002) The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat Struct Biol 9:101–106. https://doi.org/10.1038/nsb755
Heling L, Geeves MA, Kad NM (2020) MyBP-C: one protein to govern them all. J Muscle Res Cell Motil 41:91–101. https://doi.org/10.1007/s10974-019-09567-1
Hemmings L et al (1996) Talin contains three actin-binding sites each of which is adjacent to a vinculin-binding site. J Cell Sci 109(Pt 11):2715–2726
Hibberd MG, Jewell BR (1982) Calcium- and length-dependent force production in rat ventricular muscle. J Physiol 329:527–540. https://doi.org/10.1113/jphysiol.1982.sp014317
Hirt MN, Hansen A, Eschenhagen T (2014) Cardiac tissue engineering: state of the art. Circ Res 114:354–367. https://doi.org/10.1161/circresaha.114.300522
Hoshijima M (2006) Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Phys Heart Circ Phys 290:H1313–H1325. https://doi.org/10.1152/ajpheart.00816.2005
https://www.nature.com/articles/srep20674 (n.d.) - supplementary-information
Huang DL, Bax NA, Buckley CD, Weis WI, Dunn AR (2017) Vinculin forms a directionally asymmetric catch bond with F-actin. Science 357:703–706. https://doi.org/10.1126/science.aan2556
Hughes DC, Ellefsen S, Baar K (2018) Adaptations to Endurance and Strength Training. Cold Spring Harbor Perspectives in Medicine 8. https://doi.org/10.1101/cshperspect.a029769
Humphries MJ (2000) Integrin structure. Biochem Soc Trans 28:311–339. https://doi.org/10.1042/bst0280311
Huxley HE (1953) Electron microscope studies of the organisation of the filaments in striated muscle. Biochim Biophys Acta 12:387–394. https://doi.org/10.1016/0006-3002(53)90156-5
Huxley AF, Niedergerke R (1954) Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature 173:971–973. https://doi.org/10.1038/173971a0
Ingber DE (2006) Cellular mechanotransduction: putting all the pieces together again. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology 20:811–827. https://doi.org/10.1096/fj.05-5424rev
Irie T et al (2015) S-Nitrosylation of Calcium-Handling Proteins in Cardiac Adrenergic Signaling and Hypertrophy. Circ Res 117:793–803. https://doi.org/10.1161/circresaha.115.307157
Iwamoto DV, Huehn A, Simon B, Huet-Calderwood C, Baldassarre M, Sindelar CV, Calderwood DA (2018) Structural basis of the filamin A actin-binding domain interaction with F-actin. Nat Struct Mol Biol 25:918–927. https://doi.org/10.1038/s41594-018-0128-3
Iyer SR, Shah SB, Ward CW, Stains JP, Spangenburg EE, Folker ES, Lovering RM (2019) Differential YAP nuclear signaling in healthy and dystrophic skeletal muscle. Am J Physiol Cell Physiol 317:C48–c57. https://doi.org/10.1152/ajpcell.00432.2018
Izard T, Brown DT (2016) Mechanisms and Functions of Vinculin Interactions with Phospholipids at Cell Adhesion Sites. J Biol Chem 291:2548–2555. https://doi.org/10.1074/jbc.R115.686493
Izard T, Evans G, Borgon RA, Rush CL, Bricogne G, Bois PR (2004) Vinculin activation by talin through helical bundle conversion. Nature 427:171–175. https://doi.org/10.1038/nature02281
Janssens JV, Ma B, Brimble MA, Van Eyk JE, Delbridge LMD, Mellor KM (2018) Cardiac troponins may be irreversibly modified by glycation: novel potential mechanisms of cardiac performance modulation. Sci Rep 8:16084. https://doi.org/10.1038/s41598-018-33886-x
Jiang F et al (2021) The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction. Nat Commun 12:869. https://doi.org/10.1038/s41467-021-21178-4
Kamm KE, Stull JT (2011) Signaling to myosin regulatory light chain in sarcomeres. J Biol Chem 286:9941–9947. https://doi.org/10.1074/jbc.R110.198697
Kampourakis T, Sun YB, Irving M (2016) Myosin light chain phosphorylation enhances contraction of heart muscle via structural changes in both thick and thin filaments. Proc Natl Acad Sci U S A 113:E3039–E3047. https://doi.org/10.1073/pnas.1602776113
Kawai M, Jin JP (2021) Mechanisms of Frank-Starling law of the heart and stretch activation in striated muscles may have a common molecular origin. J Muscle Res Cell Motil. https://doi.org/10.1007/s10974-020-09595-2
Kehat I et al (2011) Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res 108:176–183. https://doi.org/10.1161/circresaha.110.231514
Khan RB, Goult BT (2019) Adhesions assemble!-autoinhibition as a major regulatory mechanism of integrin-mediated adhesion. Front Mol Biosci 6:144. https://doi.org/10.3389/fmolb.2019.00144
Kiema T et al (2006) The molecular basis of filamin binding to integrins and competition with talin. Mol Cell 21:337–347. https://doi.org/10.1016/j.molcel.2006.01.011
Kim K, McCully ME, Bhattacharya N, Butler B, Sept D, Cooper JA (2007) Structure/function analysis of the interaction of phosphatidylinositol 4,5-bisphosphate with actin-capping protein: implications for how capping protein binds the actin filament. J Biol Chem 282:5871–5879. https://doi.org/10.1074/jbc.M609850200
Kim T, Cooper JA, Sept D (2010) The interaction of capping protein with the barbed end of the actin filament. J Mol Biol 404:794–802. https://doi.org/10.1016/j.jmb.2010.10.017
Knöll R, Hoshijima M, Chien K (2003) Cardiac mechanotransduction and implications for heart disease. Journal of Molecular Medicine (Berlin, Germany) 81:750–756. https://doi.org/10.1007/s00109-003-0488-x
Knöll R et al (2011) Telethonin deficiency is associated with maladaptation to biomechanical stress in the mammalian heart. Circ Res 109:758–769. https://doi.org/10.1161/circresaha.111.245787
Kong Y, Shelton JM, Rothermel B, Li X, Richardson JA, Bassel-Duby R, Williams RS (2001) Cardiac-specific LIM protein FHL2 modifies the hypertrophic response to beta-adrenergic stimulation. Circulation 103:2731–2738. https://doi.org/10.1161/01.cir.103.22.2731
Konhilas JP, Irving TC, de Tombe PP (2002a) Length-dependent activation in three striated muscle types of the rat. J Physiol 544:225–236. https://doi.org/10.1113/jphysiol.2002.024505
Konhilas JP, Irving TC, de Tombe PP (2002b) Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ Res 90:59–65. https://doi.org/10.1161/hh0102.102269
Koshman YE, Piano MR, Russell B, Schwertz DW (2010) Signaling responses after exposure to 5 alpha-dihydrotestosterone or 17 beta-estradiol in norepinephrine-induced hypertrophy of neonatal rat ventricular myocytes. Journal of Applied Physiology (Bethesda, Md : 1985) 108:686–696. https://doi.org/10.1152/japplphysiol.00994.2009
Kumar M, Govindan S, Zhang M, Khairallah RJ, Martin JL, Sadayappan S, de Tombe PP (2015) Cardiac myosin-binding Protein C and Troponin-I phosphorylation independently modulate myofilament length-dependent activation. J Biol Chem 290:29241–29249. https://doi.org/10.1074/jbc.M115.686790
Lange S, Auerbach D, McLoughlin P, Perriard E, Schäfer BW, Perriard JC, Ehler E (2002) Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci 115:4925–4936. https://doi.org/10.1242/jcs.00181
Lange S et al (2005) The Kinase domain of titin controls muscle gene expression and protein turnover. Science 308:1599–1603. https://doi.org/10.1126/science.1110463
Lee HS et al (2004) Characterization of an actin-binding site within the talin FERM domain. J Mol Biol 343:771–784. https://doi.org/10.1016/j.jmb.2004.08.069
LeWinter MM, Granzier H (2010) Cardiac titin: a multifunctional giant. Circulation 121:2137–2145. https://doi.org/10.1161/circulationaha.109.860171
Li A, Ponten F, dos Remedios CG (2012) The interactome of LIM domain proteins: the contributions of LIM domain proteins to heart failure and heart development. Proteomics 12:203–225. https://doi.org/10.1002/pmic.201100492
Li J, Tanhehco EJ, Russell B (2014) Actin dynamics is rapidly regulated by the PTEN and PIP2 signaling pathways leading to myocyte hypertrophy. Am J Phys Heart Circ Phys 307:H1618–H1625. https://doi.org/10.1152/ajpheart.00393.2014
Li J, Mkrtschjan MA, Lin YH, Russell B (2016) Variation in stiffness regulates cardiac myocyte hypertrophy via signaling pathways. Can J Physiol Pharmacol 94:1–9. https://doi.org/10.1139/cjpp-2015-0578
Li J et al (2020) Signalosome-regulated serum response factor phosphorylation determining myocyte growth in width versus length as a therapeutic target for heart failure. Circulation 142:2138–2154. https://doi.org/10.1161/circulationaha.119.044805
Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ (2007) Structural basis for the autoinhibition of focal adhesion kinase. Cell 129:1177–1187. https://doi.org/10.1016/j.cell.2007.05.041
Lin YH, Li J, Swanson ER, Russell B (2013) CapZ and actin capping dynamics increase in myocytes after a bout of exercise and abates in hours after stimulation ends. Journal of Applied Physiology (Bethesda, Md : 1985) 114:1603–1609. https://doi.org/10.1152/japplphysiol.01283.2012
Lin Y-H, Swanson ER, Li J, Mkrtschjan MA, Russell B (2015) Cyclic mechanical strain of myocytes modifies CapZβ1 post translationally via PKCε. J Muscle Res Cell Motil 36:329–337. https://doi.org/10.1007/s10974-015-9420-6
Lin YH, Warren CM, Li J, McKinsey TA, Russell B (2016) Myofibril growth during cardiac hypertrophy is regulated through dual phosphorylation and acetylation of the actin capping protein CapZ. Cell Signal 28:1015–1024. https://doi.org/10.1016/j.cellsig.2016.05.011
Lin YH et al (2020) Site-specific acetyl-mimetic modification of cardiac troponin I modulates myofilament relaxation and calcium sensitivity. J Mol Cell Cardiol 139:135–147. https://doi.org/10.1016/j.yjmcc.2020.01.007
Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA (2002) Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 420:89–93. https://doi.org/10.1038/nature01082
Liu C, Kawana M, Song D, Ruppel KM, Spudich JA (2018) Controlling load-dependent kinetics of β-cardiac myosin at the single-molecule level. Nat Struct Mol Biol 25:505–514. https://doi.org/10.1038/s41594-018-0069-x
Lodka D et al (2016) Muscle RING-finger 2 and 3 maintain striated-muscle structure and function. J Cachexia Sarcopenia Muscle 7:165–180. https://doi.org/10.1002/jcsm.12057
Lorenzen-Schmidt I, Clarke SB, Pyle WG (2016) The neglected messengers: control of cardiac myofilaments by protein phosphatases. J Mol Cell Cardiol 101:81–89. https://doi.org/10.1016/j.yjmcc.2016.10.002
Lyon RC, Zanella F, Omens JH, Sheikh F (2015) Mechanotransduction in cardiac hypertrophy and failure. Circ Res 116:1462–1476. https://doi.org/10.1161/circresaha.116.304937
Manso AM et al (2013) Talin1 has unique expression versus talin 2 in the heart and modifies the hypertrophic response to pressure overload. J Biol Chem 288:4252–4264. https://doi.org/10.1074/jbc.M112.427484
Mansour H, de Tombe PP, Samarel AM, Russell B (2004) Restoration of resting sarcomere length after uniaxial static strain is regulated by protein kinase Cepsilon and focal adhesion kinase. Circ Res 94(5):642–9. https://doi.org/10.1161/01.RES.0000121101.32286.C8. Epub 2004 Feb 12
Mao Z, Nakamura F (2020) Structure and function of filamin C in the muscle Z-Disc. Int J Mol Sci 21. https://doi.org/10.3390/ijms21082696
Markert CD et al (2010) Functional muscle analysis of the Tcap knockout mouse. Hum Mol Genet 19:2268–2283. https://doi.org/10.1093/hmg/ddq105
Martin AF (1981) Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem 256:964–968. https://doi.org/10.1016/S0021-9258(19)70073-8
Mayans O et al (1998) Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature 395:863–869. https://doi.org/10.1038/27603
McKinsey TA (2012) Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol 52:303–319. https://doi.org/10.1146/annurev-pharmtox-010611-134712
Mehta AD, Rock RS, Rief M, Spudich JA, Mooseker MS, Cheney RE (1999) Myosin-V is a processive actin-based motor. Nature 400:590–593. https://doi.org/10.1038/23072
Mentes A et al (2018) High-resolution cryo-EM structures of actin-bound myosin states reveal the mechanism of myosin force sensing. Proc Natl Acad Sci U S A 115:1292–1297. https://doi.org/10.1073/pnas.1718316115
Merkel CD, Li Y, Raza Q, Stolz DB, Kwiatkowski AV (2019) Vinculin anchors contractile actin to the cardiomyocyte adherens junction. Mol Biol Cell 30:2639–2650. https://doi.org/10.1091/mbc.E19-04-0216
Miano JM, Long X, Fujiwara K (2007) Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol 292:C70–C81. https://doi.org/10.1152/ajpcell.00386.2006
Mkrtschjan MA, Solís C, Wondmagegn AY, Majithia J, Russell B (2018) PKC epsilon signaling effect on actin assembly is diminished in cardiomyocytes when challenged to additional work in a stiff microenvironment. Cytoskeleton (Hoboken, NJ) 75:363–371. https://doi.org/10.1002/cm.21472
Nakamura M, Sadoshima J (2018) Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 15:387–407. https://doi.org/10.1038/s41569-018-0007-y
Nakazawa N, Sathe AR, Shivashankar GV, Sheetz MP (2016) Matrix mechanics controls FHL2 movement to the nucleus to activate p21 expression. Proc Natl Acad Sci U S A 113:E6813–e6822. https://doi.org/10.1073/pnas.1608210113
Narita A, Takeda S, Yamashita A, Maéda Y (2006) Structural basis of actin filament capping at the barbed-end: a cryo-electron microscopy study. EMBO J 25:5626–5633. https://doi.org/10.1038/sj.emboj.7601395
Narolska NA et al (2006) Impaired diastolic function after exchange of endogenous troponin I with C-terminal truncated troponin I in human cardiac muscle. Circ Res 99:1012–1020. https://doi.org/10.1161/01.RES.0000248753.30340.af
Oda T, Yanagisawa H (2020) Cryo-electron tomography of cardiac myofibrils reveals a 3D lattice spring within the Z-discs. Commun Biol 3:585. https://doi.org/10.1038/s42003-020-01321-5
Olson EN, Nordheim A (2010) Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol 11:353–365. https://doi.org/10.1038/nrm2890
Pandey P et al (2018) Cardiomyocytes sense matrix rigidity through a combination of muscle and non-muscle myosin contractions. Dev Cell 44:326–336.e323. https://doi.org/10.1016/j.devcel.2017.12.024
Pfleger J, Gresham K, Koch WJ (2019) G protein-coupled receptor kinases as therapeutic targets in the heart. Nat Rev Cardiol 16:612–622. https://doi.org/10.1038/s41569-019-0220-3
Pitoulis FG et al (2021) Remodelling of adult cardiac tissue subjected to physiological and pathological mechanical load in vitro. Cardiovasc Res. https://doi.org/10.1093/cvr/cvab084
Planelles-Herrero VJ, Hartman JJ, Robert-Paganin J, Malik FI, Houdusse A (2017) Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil. Nat Commun 8:190. https://doi.org/10.1038/s41467-017-00176-5
Puchner EM et al (2008) Mechanoenzymatics of titin kinase. Proc Natl Acad Sci U S A 105:13385–13390. https://doi.org/10.1073/pnas.0805034105
Pudas R, Kiema TR, Butler PJ, Stewart M, Ylänne J (2005) Structural basis for vertebrate filamin dimerization. Structure (London, England : 1993) 13:111–119. https://doi.org/10.1016/j.str.2004.10.014
Purcell NH, Darwis D, Bueno OF, Müller JM, Schüle R, Molkentin JD (2004) Extracellular signal-regulated kinase 2 interacts with and is negatively regulated by the LIM-only protein FHL2 in cardiomyocytes. Mol Cell Biol 24:1081–1095. https://doi.org/10.1128/mcb.24.3.1081-1095.2004
Pyle WG, La Rotta G, de Tombe PP, Sumandea MP, Solaro RJ (2006) Control of cardiac myofilament activation and PKC-betaII signaling through the actin capping protein, CapZ. J Mol Cell Cardiol 41(3):537–43. https://doi.org/10.1016/j.yjmcc.2006.06.006
Radke MH, Peng J, Wu Y, McNabb M, Nelson OL, Granzier H, Gotthardt M (2007) Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci U S A 104:3444–3449. https://doi.org/10.1073/pnas.0608543104
Razinia Z, Mäkelä T, Ylänne J, Calderwood DA (2012) Filamins in mechanosensing and signaling. Annu Rev Biophys 41:227–246. https://doi.org/10.1146/annurev-biophys-050511-102252
Reconditi M et al (2017) Myosin filament activation in the heart is tuned to the mechanical task. Proc Natl Acad Sci U S A 114:3240–3245. https://doi.org/10.1073/pnas.1619484114
Reinoso TR et al (2020) A comprehensive guide to genetic variants and post-translational modifications of cardiac troponin C. J Muscle Res Cell Motil. https://doi.org/10.1007/s10974-020-09592-5
Rhee D, Sanger JM, Sanger JW (1994) The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil Cytoskeleton 28:1–24. https://doi.org/10.1002/cm.970280102
Ribeiro Ede A Jr, Pinotsis N, Ghisleni A, Salmazo A, Konarev PV, Kostan J, et al (2014) The structure and regulation of human muscle α-actinin. Cell 159(6):1447–60. https://doi.org/10.1016/j.cell.2014.10.056. Epub 2014 Nov 26.
Rudolph F et al (2019) Resolving titin's lifecycle and the spatial organization of protein turnover in mouse cardiomyocytes. Proc Natl Acad Sci U S A 116:25126–25136. https://doi.org/10.1073/pnas.1904385116
Russell B, Solís C (2021) Mechanosignaling pathways alter muscle structure and function by post-translational modification of existing sarcomeric proteins to optimize energy usage. J Muscle Res Cell Motil. https://doi.org/10.1007/s10974-021-09596-9
Russell B, Wenderoth MP, Goldspink PH (1992) Remodeling of myofibrils: subcellular distribution of myosin heavy chain mRNA and protein. Am J Phys 262:R339–R345. https://doi.org/10.1152/ajpregu.1992.262.3.R339
Russell B, Motlagh D, Ashley WW (2000) Form follows function: how muscle shape is regulated by work. Journal of Applied Physiology (Bethesda, Md : 1985) 88:1127–1132. https://doi.org/10.1152/jappl.2000.88.3.1127
Russell B, Curtis MW, Koshman YE, Samarel AM (2010) Mechanical stress-induced sarcomere assembly for cardiac muscle growth in length and width. J Mol Cell Cardiol 48:817–823. https://doi.org/10.1016/j.yjmcc.2010.02.016
Ryba DM, Li J, Cowan CL, Russell B, Wolska BM, Solaro RJ (2017) Long-term biased beta-arrestin signaling improves cardiac structure and function in dilated cardiomyopathy. Circulation 135:1056–1070. https://doi.org/10.1161/circulationaha.116.024482
Ryder DJ, Judge SM, Beharry AW, Farnsworth CL, Silva JC, Judge AR (2015) Identification of the Acetylation and Ubiquitin-Modified Proteome during the Progression of Skeletal Muscle Atrophy. PLoS ONE 10:e0136247. https://doi.org/10.1371/journal.pone.0136247
Samarel AM (2014) Focal adhesion signaling in heart failure. Pflugers Arch - Eur J Physiol 466:1101–1111. https://doi.org/10.1007/s00424-014-1456-8
Samarel AM, Spragia ML, Maloney V, Kamal SA, Engelmann GL (1992) Contractile arrest accelerates myosin heavy chain degradation in neonatal rat heart cells. Am J Phys 263:C642–C652. https://doi.org/10.1152/ajpcell.1992.263.3.C642
Samarel AM, Koshman Y, Swanson ER, Russell B (2013) Biophysical forces modulate the costamere and Z-Disc for sarcomere remodeling in heart failure. In: Solaro RJ, Tardiff JC (eds) Biophysics of the failing heart: physics and biology of heart muscle. Springer New York, New York, pp 141–174. https://doi.org/10.1007/978-1-4614-7678-8_7
Samson T et al (2004) The LIM-only proteins FHL2 and FHL3 interact with alpha- and beta-subunits of the muscle alpha7beta1 integrin receptor. J Biol Chem 279:28641–28652. https://doi.org/10.1074/jbc.M312894200
Sánchez-Martín P, Komatsu M (2018) p62/SQSTM1 - steering the cell through health and disease. J Cell Sci 131. https://doi.org/10.1242/jcs.222836
Sanger JW, Wang J, Fan Y, White J, Sanger JM (2010) Assembly and dynamics of myofibrils. J Biomed Biotechnol 2010:858606. https://doi.org/10.1155/2010/858606
Sarker M et al (2019) Cardiomyopathy mutations in metavinculin disrupt regulation of vinculin-induced F-Actin assemblies. J Mol Biol 431:1604–1618. https://doi.org/10.1016/j.jmb.2019.02.024
Sellers JR (2000) Myosins: a diverse superfamily. Biochim Biophys Acta 1496:3–22. https://doi.org/10.1016/s0167-4889(00)00005-7
Senyo SE, Koshman YE, Russell B (2007) Stimulus interval, rate and direction differentially regulate phosphorylation for mechanotransduction in neonatal cardiac myocytes. FEBS Lett 581:4241–4247. https://doi.org/10.1016/j.febslet.2007.07.070
Seong J et al (2013) Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins. Proc Natl Acad Sci U S A 110:19372–19377. https://doi.org/10.1073/pnas.1307405110
Sequeira V, van der Velden J (2017) The Frank-Starling Law: a jigsaw of titin proportions. Biophys Rev 9:259–267. https://doi.org/10.1007/s12551-017-0272-8
Simpson DG, Sharp WW, Borg TK, Price RL, Terracio L, Samarel AM (1996) Mechanical regulation of cardiac myocyte protein turnover and myofibrillar structure. Am J Phys 270:C1075–C1087. https://doi.org/10.1152/ajpcell.1996.270.4.C1075
Sjöblom B, Salmazo A, Djinović-Carugo K (2008) α-Actinin structure and regulation. Cell Mol Life Sci 65:2688. https://doi.org/10.1007/s00018-008-8080-8
Solaro RJ, Henze M, Kobayashi T (2013) Integration of troponin I phosphorylation with cardiac regulatory networks. Circ Res 112:355–366. https://doi.org/10.1161/circresaha.112.268672
Solís C, Russell B (2019) CapZ integrates several signaling pathways in response to mechanical stiffness. J Gen Physiol 151:660–669. https://doi.org/10.1085/jgp.201812199
Solís C, Solaro RJ (2021) Novel insights into sarcomere regulatory systems control of cardiac thin filament activation. J Gen Physiol 153. https://doi.org/10.1085/jgp.202012777
Solis C, Kim GH, Moutsoglou ME, Robinson JM (2018) Ca(2+) and myosin cycle states work as allosteric effectors of troponin activation. Biophys J 115:1762–1769. https://doi.org/10.1016/j.bpj.2018.08.033
Sun X et al (2020) Mechanosensing through direct binding of tensed F-Actin by LIM domains. Dev Cell 55:468–482.e467. https://doi.org/10.1016/j.devcel.2020.09.022
Sung J et al (2015) Harmonic force spectroscopy measures load-dependent kinetics of individual human β-cardiac myosin molecules. Nat Commun 6:7931. https://doi.org/10.1038/ncomms8931
Suphamungmee W, Nakamura F, Hartwig JH, Lehman W (2012) Electron microscopy and 3D reconstruction reveals filamin Ig domain binding to F-actin. J Mol Biol 424:248–256. https://doi.org/10.1016/j.jmb.2012.09.025
Takeda S et al (2010) Two distinct mechanisms for actin capping protein regulation--steric and allosteric inhibition. PLoS Biol 8:e1000416. https://doi.org/10.1371/journal.pbio.1000416
Taneja N, Neininger AC, Burnette DT (2020) Coupling to substrate adhesions drives the maturation of muscle stress fibers into myofibrils within cardiomyocytes. Mol Biol Cell 31:1273–1288. https://doi.org/10.1091/mbc.E19-11-0652
Tapial Martínez P, López Navajas P, Lietha D (2020) FAK structure and regulation by membrane interactions and force in focal adhesions. Biomolecules 10. https://doi.org/10.3390/biom10020179
Torsoni AS, Constancio SS, Nadruz W Jr, Hanks SK, Franchini KG (2003) Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res 93:140–147. https://doi.org/10.1161/01.res.0000081595.25297.1b
Tripet B, Van Eyk JE, Hodges RS (1997) Mapping of a second actin-tropomyosin and a second troponin C binding site within the C terminus of troponin I, and their importance in the Ca2+-dependent regulation of muscle contraction. J Mol Biol 271:728–750. https://doi.org/10.1006/jmbi.1997.1200
Urciuoli E, Peruzzi B (2020) Involvement of the FAK network in pathologies related to altered mechanotransduction. Int J M Sci 21. https://doi.org/10.3390/ijms21249426
Van Eyk JE, Strauss JD, Hodges RS, Rüegg JC (1993) A synthetic peptide mimics troponin I function in the calcium-dependent regulation of muscle contraction. FEBS Lett 323:223–228. https://doi.org/10.1016/0014-5793(93)81344-Y
Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW (2005) Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil Cytoskeleton 61:34–48. https://doi.org/10.1002/cm.20063
Wang Y, Yan J, Goult BT (2019) Force-dependent binding constants. Biochemistry 58:4696–4709. https://doi.org/10.1021/acs.biochem.9b00453
Wang J, Fan Y, Dube S, Agassy NW, Dube DK, Sanger JM, Sanger JW (2020) Myofibril assembly and the roles of the ubiquitin proteasome system. Cytoskeleton (Hoboken, NJ) 77:456–479. https://doi.org/10.1002/cm.21641
Wang Z, Grange M, Wagner T, Kho AL, Gautel M, Raunser S (2021) The molecular basis for sarcomere organization in vertebrate skeletal muscle. Cell 184:2135–2150.e2113. https://doi.org/10.1016/j.cell.2021.02.047
Ward M, Iskratsch T (2020) Mix and (mis-)match - The mechanosensing machinery in the changing environment of the developing, healthy adult and diseased heart. Biochim Biophys Acta, Mol Cell Res 2020:118436. https://doi.org/10.1016/j.bbamcr.2019.01.017
Wear MA, Cooper JA (2004) Capping protein: new insights into mechanism and regulation. Trends Biochem Sci 29:418–428. https://doi.org/10.1016/j.tibs.2004.06.003
Wear MA, Yamashita A, Kim K, Maéda Y, Cooper JA (2003) How capping protein binds the barbed end of the actin filament. Curr Biol 13:1531–1537. https://doi.org/10.1016/s0960-9822(03)00559-1
Weis WI, Kobilka BK (2018) The Molecular Basis of G Protein-Coupled Receptor Activation. Annu Rev Biochem 87:897–919. https://doi.org/10.1146/annurev-biochem-060614-033910
Winkelman JD, Anderson CA, Suarez C, Kovar DR, Gardel ML (2020) Evolutionarily diverse LIM domain-containing proteins bind stressed actin filaments through a conserved mechanism. Proc Natl Acad Sci U S A 117:25532–25542. https://doi.org/10.1073/pnas.2004656117
Witt S, Zieseniss A, Fock U, Jockusch BM, Illenberger S (2004) Comparative biochemical analysis suggests that vinculin and metavinculin cooperate in muscular adhesion sites. J Biol Chem 279:31533–31543. https://doi.org/10.1074/jbc.M314245200
Witt SH, Granzier H, Witt CC, Labeit S (2005) MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol 350:713–722. https://doi.org/10.1016/j.jmb.2005.05.021
Witt CC, Witt SH, Lerche S, Labeit D, Back W, Labeit S (2008) Cooperative control of striated muscle mass and metabolism by MuRF1 and MuRF2. EMBO J 27:350–360. https://doi.org/10.1038/sj.emboj.7601952
Woody MS, Greenberg MJ, Barua B, Winkelmann DA, Goldman YE, Ostap EM (2018) Positive cardiac inotrope omecamtiv mecarbil activates muscle despite suppressing the myosin working stroke. Nat Commun 9:3838. https://doi.org/10.1038/s41467-018-06193-2
Yamada Y, Namba K, Fujii T (2020) Cardiac muscle thin filament structures reveal calcium regulatory mechanism. Nat Commun 11:153. https://doi.org/10.1038/s41467-019-14008-1
Yamashita A, Maeda K, Maéda Y (2003) Crystal structure of CapZ: structural basis for actin filament barbed end capping. EMBO J 22:1529–1538. https://doi.org/10.1093/emboj/cdg167
Yang C, Zhang X, Guo Y, Meng F, Sachs F, Guo J (2015) Mechanical dynamics in live cells and fluorescence-based force/tension sensors. Biochim Biophys Acta 1853:1889–1904. https://doi.org/10.1016/j.bbamcr.2015.05.001
Yang H et al (2016) Dynamic Myofibrillar Remodeling in Live Cardiomyocytes under Static Stretch. Sci Rep 6:20674. https://doi.org/10.1038/srep20674
Yao M, Goult BT, Chen H, Cong P, Sheetz MP, Yan J (2014) Mechanical activation of vinculin binding to talin locks talin in an unfolded conformation. Sci Rep 4:4610. https://doi.org/10.1038/srep04610
Yu JG, Russell B (2005) Cardiomyocyte remodeling and sarcomere addition after uniaxial static strain in vitro. The Journal of Histochemistry and Cytochemistry : Official Journal of the Histochemistry Society 53:839–844. https://doi.org/10.1369/jhc.4A6608.2005
Zhang Y et al (2019) Cardiomyocyte PKA ablation enhances basal contractility while eliminates cardiac β-Adrenergic response without adverse effects on the heart. Circ Res 124:1760–1777. https://doi.org/10.1161/circresaha.118.313417
Zou P et al (2006) Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature 439:229–233. https://doi.org/10.1038/nature04343
Acknowledgements
This work was supported by the National Institutes of Health grants HL62426 (to B. Russell, Project 2) and HL151825 (to C. Solís). The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Solís, C., Russell, B. Striated muscle proteins are regulated both by mechanical deformation and by chemical post-translational modification. Biophys Rev 13, 679–695 (2021). https://doi.org/10.1007/s12551-021-00835-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-021-00835-4