Abstract
CGA-N46 is a small antifungal-derived peptide and consists of the 31st–76th amino acids of the N-terminus of human chromogranin A. Polycistronic expression of recombinant CGA-N46 in Bacillus subtilis DB1342 was used to improve its production, but the yield of CGA-N46 was still low. In the present study, response surface methodology (RSM) was used to optimize culture medium composition and growth conditions of the engineered strain B. subtilis DB1342(p-3N46) for the further increase in CGA-N46 yield. The results of two-level factorial experiments indicated that dextrin and tryptone were significant factors affecting CGA-N46 expression. Central composite design (CCD) was used to determine the ideal conditions of each significant factors. From the results of CCD, the optimal medium composition was predicted to be dextrin 16.6 g/L, tryptone 19.2 g/L, KH2PO4·H2O 6 g/L, pH 6.5. And the optimal culture process indicated inoculation of B. subtilis DB1342(p-3N46) seed culture into fresh culture medium at 5 % (v/v), followed by expression of CGA-N46 for 56 hours at 30 °C induced by 2 % (v/v) sucrose after one hour of shaking culture. To test optimal CGA-N46 peptide expression, the yeast growth inhibition assay was employed and it was found that under optimal culture conditions, CGA-N46 inhibited the growth of Candida albican by 42.17, 30.86 % more than that in the pre-optimization conditions. In summary, RSM can be used to optimize expression conditions of CGA-N46 in engineered strains B. subtilis DB1342(p-3N46).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Candida albican, a ubiquitously distributed opportunistic pathogen, is the leading cause of candidas and causes one of the highest numbers of deaths among patients with fungal infections in the world [1, 2, 3]. Azole drugs are commonly used to treat infections caused by C. albican. However, some Candida spp. are intrinsically resistant or have reduced susceptibility to antifungal agents [4, 5, 6]. Antimicrobial peptides (AMPs) are multidimensionality powerful host shield and are hard for microorganisms to overcome using single approach resistance strategies. AMPs have shown to be effective alternatives to the current antimycotic therapies with the increasing resistance to conventional antimycotic drugs [7, 8]. CGA-N46, a peptide containing the 31st–76th amino acid of human chromogranin A, has antagonistic activity to C. albican [9]. To meet the need for drug development for future clinical applications, an abundance of CGA-N46 is necessary.
There are numerous approaches to optimizing recombinant expression. They are mainly two classes, i.e., genetic engineering and culture conditions optimization. The approaches on high efficient expression optimization mainly were genetic engineering, such as fusion expression [10, 11], induced expression [12, 13], or targeted codon optimization [14]. Culture conditions optimization were conducted in high-density cultivation [13, 15]. Li et al. [16] previously constructed an inducible tri-cistronic expression plasmid p-3N46 containing CGA-N46, which allowed three copies of cga-N46 gene be induced to express in one plasmid. Using this genetic strain, the expression of cga-N46 gene was increased by optimizing plasmid.
Production yield and cost of recombinant proteins are considerably influenced by bacterial culture conditions and medium composition. Optimization of culture conditions using biostatistic methods is another method to increase the expression of an engineered strain. Because of the difficulty of purification, one-factor-at-a-time was usually used [17]. However, this method frequently failed to locate the region of optimal response. Response surface methodology (RSM), exploring the relationships between several explanatory variables and one or more response variables [18], is characterized by more variables, fewer experiments, shorter cycle, and higher accuracy than other statistical optimization techniques such as one-factor-at-a-time [19, 17] or orthogonal tests. The main idea of RSM is to use a sequence of designed experiments to obtain an optimal response by using a second-degree polynomial model. As an optimization method, the experimental random error is considered in RSM, which is not in other traditional biostatistic methods. RSM is a simple, useful, and more precise methodology that can determine optimal culture medium composition and culture conditions using biostatistic computer software. RSM is commonly used for enhancing microorganism metabolite production at present [20, 21, 22, 18]. However, it is rarely reported in engineered strain expression.
In this study, RSM was used to optimize the medium and culture conditions for maximal expression of CGA-N46. To quantify CGA-N46, its effectiveness on inhibiting C. albican growth were tested.
2 Methods
2.1 Microorganisms
The engineered strains B. subtilis DB1342(pSBPTQ) which bore plasmid pSBPTQ [23] and B. subtilis DB1342(p-3N46) which bore plasmid p-3N46 [17] used in this study were previously constructed.
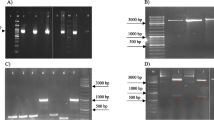
Plasmid pSBPTQ, an Escherichia coli and Bacillus subtilis shuttle plasmid, was a cloning plasmid with ampicillin resistance (Amp r) in E. coli and an expression plasmid with kanamycin resistance (Kan r) in B. subtilis. It had sacB promoter sequence (sacB ps) which can be induced by sucrose and promote the transcription of the exogenous gene downstream of sacB ps. It had multicloning sites between sacB ps and terminator sequence (T) which allowed inserting exogenous gene in pSBPTQ. The terminator could make an efficient stop of the transcription. Plasmid p-3N46 was a recombinant vector from pSBPTQ which bore three cistrons of cga-N46 gene between sacB ps and terminator in pSBPTQ. Each cga-N46 cistron had one ribosome binding site, one initiating codon, one copy of cga-N46 gene, one stop codon. Three cistrons of cga-N46 gene allowed three copies of CGA-N46 to be expressed in a single plasmid p-3N46. Their physical maps are shown in Fig. 1.
Yeast strain C. albican CSC314 was supplied by the Institute of Dermatology, Chinese Academy of Medical Sciences.
2.2 Culture Media and Growth Conditions
Luria-Bertani (LB) agar medium (10 g tryptone, 5 g yeast extract, 10 g NaCl, 18 g agar in 1000 mL of distilled water, pH 7.0) containing 10 μg/mL kanamycin was used to culture B. subtilis DB1342 (pSBPTQ) and B. subtilis DB1342(p-3N46). The LB broth medium was prepared with the components as LB agar medium except agar as the growing culture for bacteria.
2 × MSR medium [24], contained glucose 10 g, tryptone 30 g, yeast extract 50 g, KH2PO4 6 g in 1000 mL of distilled water, was used for expression culture optimization studies.
YPD medium (yeast extract 10 g, peptone 20 g, dextrose 20 g in 1000 mL of distilled water) was used to culture C. albican CSC314.
The seed culture was prepared by transferring a loopful of fresh transformed strains B. subtilis DB1342(pSBPTQ) and B. subtilis DB1342(p-3N46) cells into 25 ml LB broth in 250 mL Erlenmeyer flask. The flask was incubated at 250 rpm at 37 °C for 16 h.
2.3 Expression Conditions
The production process was carried out, unless otherwise mentioned, in Erlenmeyer flasks (500 mL) containing 100 mL aliquots of the basal culture medium with 10 μg/mL kanamycin. After cultivated for 2 h at 37 °C 200 rpm, 5 mL 40 % sterile sucrose solution was added into culture medium to induce the expression of CGA-N46 for 48 h. During this period, CGA-N46 was expressed and secreted into culture supernatant. After expression, both of the culture were centrifuged at 12,000×g for 10 min at 4 °C. For antagonistic activity determination, 10 mL culture supernatant was filtered with 0.22 μm filter film.
2.4 Assay of Antagonistic Activity
To analyze the antagonistic activity, 10 mL supernatant was mixed with equal volume of sterile YPD medium. C. albican (106 cells/mL) was inoculated into the mixture medium at 10 % (v/v) and then incubated at 26 °C, 200 rpm. The supernatant from B. subtilis DB1342(pSBPTQ) was used as control. After incubating for 24 h, the yeast cells were centrifuged at 10,000×g for 10 min at room temperature. The cell precipitates were dried in a constant temperature oven at 37 °C to remove all traces of water. The dried cell precipitates were weighed, and yeast growth inhibition caused by CGA-N46 was calculated according to Eq. (1).
Here, W 1 was the yeast cell dry weight in experimental group, W 2 was the yeast cell dry weight in control group, and Y was the indicator of yeast growth inhibition rate.
All experiments were conducted in triplicate, and the mean values were reported with standard error.
2.5 Carbon Source Screening
The carbon source in \(2\times \hbox {MSR}\) medium was replaced by glycerol, maltose, lactose, dextrin, and soluble starch, respectively, to optimize the carbon source of the culture medium. B. subtilis DB1342(p-3N46) seed culture was inoculated into the carbon source modified \(2\times \hbox {MSR}\) medium to express CGA-N46. Yeast growth inhibition rates of B. subtilis DB1342(p-3N46) culture supernatant from each medium were measured.
2.6 Nitrogen Source Screening
To study the effect of nitrogen source of the culture medium on the antagonistic activity of culture supernatant, the nitrogen source in \(2\times \hbox {MSR}\) medium was replaced by casein, peptone, KNO3, ammonium sulfate, and urea, respectively. B. subtilis DB1342(p-3N46) seed culture was inoculated into nitrogen source modified \(2\times \hbox {MSR}\) medium to express CGA-N46. Yeast growth inhibition rates of B. subtilis DB1342(p-3N46) culture supernatant from each medium were measured.
2.7 Computer Software for Experimental Design and Statistical Analysis
The computer software, Design Expert 7.1 (Stat-Ease, Inc, Minneapolis, MN), was used for experimental design and regression analysis of the experimental data. For analysis of the fitted response nature and prediction of the maximum point, second-order equation was reduced to its canonical form.
2.8 Response Surface Methodology for Optimizing Culture Conditions
2.8.1 Two-Level Factorial Experiment
Two-level factorial experiments were used to find significant factors. Considering the culture conditions reported in previous studies, the main factors in this study were set as follow (as shown in Table 1): dextrin (A), tryptone (B), initial medium pH (C), seed culture inoculation concentration (D), sucrose added time (E), induction period (F), and induction temperature (G). Design Expert 7.1 was used to design the experiments. Yeast growth inhibition rate (Y) was designed as response values. The significant factors affecting the expression of CGA-N46 would be determined according to the response values of different experimental conditions. Each experiment was conducted in three replicates.
2.8.2 The Steepest Descent Experiment
In RSM, the optimal area of single significant factors could be further explored by designing the steepest descent experiments based on the results of two-level factorial experiments. It is also why RSM has higher accuracy than other statistical optimization techniques. These experimental designs are shown in Table 3. The optimal area of significant factors would be determined based on the response values.
2.8.3 Central Composite Design
In RSM, after the steepest ascent path experiments of single significant factors, the optimal level of significant factors would be further studied by central composite experiments. Central composite experiment design is shown in Table 4.
3 Results and Discussion
3.1 Effect of Carbon Source on Yeast Growth Inhibition Rate
Yeast growth inhibition rates of B. subtilis DB1342(p-3N46) culture supernatant from each carbon source modified \(2\times \hbox {MSR}\) media were calculated, and the results are presented in Fig. 2. The maximum yeast growth inhibition rate was 12.23 % in dextrin modified \(2\times \hbox {MSR}\) medium, which increased 1.59 % than that of the control (10.64 %). Therefore, dextrin was the optimal carbon source for CGA-N46 expression.
3.2 Effect of Nitrogen Source on Yeast Growth Inhibition Rate
Yeast growth inhibition rates of B. subtilis DB1342(p-3N46) culture supernatant from each nitrogen source modified \(2\times \hbox {MSR}\) media were calculated, and the results are shown in Fig. 3. The maximum yeast growth inhibition rate was 11.07 % with tryptone as nitrogen resource (the control). Tryptone was chosen to be the optimal nitrogen source for CGA-N46 expression.
3.3 Yeast Growth Inhibition Rate in Pre-optimization Conditions
From the results of one-factor-at-a-time method for screening carbon and nitrogen sources, the culture medium composition was obtained as follows: yeast extract 50 g/L, dextrin 10 g/L, tryptone 30 g/L, KH2PO4·H2O 6 g/L. This culture medium was considered as pre-optimization medium. The antagonistic activity of B. subtilis DB1342(p-3N46) culture supernatant in pre-optimization conditions was calculated to be 11.31 %.
3.4 Significant Factors
There were 20 different experiments designed by software Design Expert 7.1 for two-level factorial experiment. The experiment results (i.e., response values) are shown in Table 1. The main effect analysis of the influencing factors is shown in Table 2. Dextrin and tryptone were two statistically significant factors (p < 0.05) that affected the yeast growth inhibition rate of B. subtilis DB1342(p-3N46) culture supernatant.
The multiple regression equation of the response values (yeast growth inhibition rate) for the independent variables was obtained by software analysis.
In Eq. (2), the independent variables included dextrin (A), tryptone (B), medium pH (C), seed culture inoculation concentration (D), sucrose added time (E), induction period (F), and induction temperature (G). From the coefficients of different variables in Eq. (2), the statistically nonsignificant factors, including medium pH, seed culture inoculation concentration, sucrose added time, and induction temperature were negative factors for response value, while the statistically nonsignificant factor induction period was positive one. Therefore, the −1 level for negative factors and the +1 level for positive factor were chosen for subsequent experiments. The values of the statistically nonsignificant factors were as follows: pH 6.5, the seed culture inoculation concentration 5 % (v/v), sucrose added after the seed culture inoculated for 1 h, induction temperature 30 °C, and induction period 56 h.
3.5 The Steepest Descent Path Test Results of Significant Factors
The coefficients of significant factors dextrin (A) and tryptone (B) in Eq. (2) suggested that reducing the concentrations of dextrin and tryptone could improve the response value (yeast growth inhibition rate). The steepest descent path tests of single factor were designed to further optimize the significant factors. As initiation concentration of the descent path, 40 g/L dextrin and tryptone were chosen , and 5 g/L was set as the step length. The results in Table 3 showed that the response value of B. subtilis DB1342(p-3N46) supernatant in run 6 (dextrin 15 g/L and tryptone 15 g/L) was 41.11 %, suggesting that the optimal concentrations of dextrin and tryptone for response value were near 15 g/L.
3.6 Central Composite Experimental Results
To get the exact optimal concentrations of significant factors, central composite experiments were performed. 15 g/L dextrin and 15 g/L tryptone were set as the central value. The levels of dextrin and tryptone and central composite experiment designs are shown in Table 4. A regression equation was obtained for response value when RSM analysis was used to evaluate dextrin and tryptone variables.
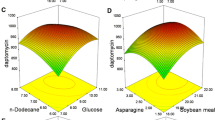
The variance of regression equation was analyzed by software Design Expert 7.10. The results are shown in Table 5 and Fig. 4.
The interaction effect of significant variables (dextrin and tryptone) on the response value (yeast growth inhibition rate) was studied by plotting three-dimensional surface curve. The three-dimensional curve of the calculated response (yeast growth inhibition rate) and contour plot from the interaction between the variables are shown in Fig. 4. The optimal concentration of dextrin and tryptone were 16.6 and 19.2 g/L, respectively. The theoretical maximum value of the yeast growth inhibition rate of DB1342(p-3N46) culture supernatant was 43.98 %.
3.7 Validation of Experimental Model
Bacillus subtilis DB1342(p-3N46) and DB1342(pSBPTQ) were cultured under the optimized conditions. The yeast growth inhibition rate of B. subtilis DB1342(p-3N46) supernatant was 42.17 %, which was very close to the theoretical result 43.98 %. The result suggested that the experimental model was quite accurate. The results also showed that the inhibition rate of DB1342(p-3N46) supernatant from optimal conditions was significantly increased compared to the original conditions.
Compared with genetic engineering, the enhanced yield of CGA-N46 in culture conditions optimization was less, but it could be a valuable compensation to genetic engineering. Moreover, culture conditions optimization is a method to reduce the cost of expression for CGA-N46 industrial development.
This paper focused on investigation of significant factors that were responsible for peptide CGA-N46 expression in B. subtilis engineered strain DB1342(p-3N46) using mathematical and computational methods. The factors, such as nitrogen source and carbon source of medium components, initial medium pH, inoculum size, sucrose added time, and induction period, were considered. Although more factors were considered in this investigation than in other reports [25, 26], some other factors, such as metallic ions, could further improve engineered strain’s expression. But for the facilitation of the purification, the medium needs to be as simple as possible. Therefore, only some basal culture factors were chosen as the variables in the present investigation.
4 Conclusion
Response surface methodology (RSM) experimental designs offered an efficient and feasible approach for culture conditions optimization of CGA-N46 expression in B. subtilis DB1342(p-3N46). The optimal medium composition was yeast 50 g/L, dextrin 16.6 g/L, tryptone 19.2 g/L, KH2PO4·H2O 6 g/L, pH6.5. The optimal culture conditions were inoculating seed culture at concentration of 5 % (v/v), adding sucrose up to 2 % one hour after the seed culture was inoculated, and allowing cultures to incubate at 30 °C for 56 h. Under optimal culture conditions, yeast growth inhibition rate of CGA-N46 was 31.53 % higher than that in original conditions. It was concluded that RSM was a method for efficiently improving the production of CGA-N46 in the engineered strain B. subtilis DB1342(p-3N46) and might provide an alternative approach to enhance the recombinant protein productivity in other engineered strains.
References
Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White T (2012) Hidden killers: human fungal infections. Sci Transl Med 4:165rv13
Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163
Pfaller MA, Diekema DJ (2010) Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36:1–53
Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C (2011) Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS one 6:e24198
Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, Carlet J, Reynes J, Rosenheim M, Regnier B, Lortholary O, Amar Cand Study Group (2009) Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med 37:1612–1618
Scorzoni L, de Lucas MP, Mesa-Arango AC, Fusco-Almeida AM, Lozano E, Cuenca-Estrella M, Mendes-Giannini MJ, Zaragoza O (2013) Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS one 8:e60047
Foubister V (2003) Superpeptide to treat Candida albicans. Drug Discov Today 8:380–381
Silva PM, Gonçalves S, Santos NC (2014) Defensins: antifungal lessons from eukaryotes. Frontiers Microbiol 5:1
Li R, Zhang T, Luo J (2006) Construction of an inducible and efficient expression secretion shuttle vector of B. subtilis. Acta Microbiol Sin 46:714–719 (in Chinese)
Wu D, Lu Y, Huang H, Ma L, Che Y, Zha X, Yao B, Yang P (2013) High-level secretory expression of metchnikowin in Escherichia coli. Protein Expres Purif 91:49–53
Xie YG, Han FF, Luan C, Zhang HW, Feng J, Choi YJ, Groleau D, Wang YZ (2013) High-yield soluble expression and simple purification of the antimicrobial peptide OG2 using the intein system in Escherichia coli. BioMed Res Int. doi:10.1155/2013/754319
Fan L, Kadura I, Krebs LE, Larson JL, Bowden DM, Frye CC (2013) Development of a highly-efficient CHO cell line generation system with engineered SV40E promoter. J Biotechnol 168:652–658
Nie Y, Yan W, Xu Y, Chen WB, Mu XQ, Wang X, Xiao R (2013) High-level expression of Bacillus naganoensis pullulanase from recombinant Escherichia coli with auto-induction: effect of lac operator. PLoS one 8:e78416
Hutterer KM, Zhang Z, Michaels ML, Belouski E, Hong RW, Shah B, Berge M, Barkhordarian H, Le E, Smith S, Winters D, Abroson F, Hecht R, Liu J (2012) Targeted codon optimization improves translational fidelity for an Fc fusion protein. Biotechnol Bioeng 109:2770–2777
Peng Z, Wang A, Feng Q, Wang Z, Ivanova IV, He X, Zhang B, Song W (2014) High-level expression, purification and characterization of porcine \(\beta\)-defensin 2 in Pichia pastoris and its potential as a cost-efficient growth promoter in porcine feed. Appl Microbiol Biotechnol 98:5487–5497
Li R, Wang B, Yi Y, Huang L, Xiong Q (2013) Polycistronic expression of CGA-N46 gene in Bacillus subtilis DB1342. Afr J Microbiol Res 7:3294–3303
Zarschler K, Witecy S, Kapplusch F, Foerster C, Stephan H (2013) High-yield production of functional soluble single-domain antibodies in the cytoplasm of Escherichia coli. Microb Cell Fact 12:97
Zhao X, Han Y, Tan X, Wang J, Zhou Z (2014) Optimization of antifungal lipopeptide production from Bacillus sp. BH072 by response surface methodology. J Microbiol 52:324–332
Givry S, Duchiron F (2008) Optimization of culture medium and growth conditions for production of L-arabinose isomerase and D-xylose isomerase by Lactobacillus bifermentans. Microbiology 77:281–287
He S, Wang H, Wu B, Zhou H, Zhu P, Yang R, Yan X (2013) Response surface methodology optimization of fermentation conditions for rapid and efficient accumulation of macrolactin A by marine Bacillus amyloliquefaciens ESB-2. Molecules 18:408–417
Li R, Xu Y (2011) Fermentation optimization to improve the production of antagonistic metabolites by Bacillus subtilis strain BS501a. J Cent South Univ Technol 18:1047–1053
Su X, Liu Y, Hu J, Ding L, Shen C (2014) Optimization of protein production by Micrococcus luteus for exploring pollutant-degrading uncultured bacteria. SpringerPlus 3:117
Li R, Zhang T, Luo J, Wang F, Gu Q, Gan J, Xiao F (2006) Antifungal activity fragments of N domain of chromogranin A. Acta Sci Nat Univ Sunyatseni 45:64–67 (in Chinese)
Ye RQ, Kim JH, Kim BG, Szarka S, Sihota E, Wong SL (1999) High-level secretory production of intact, biologically active staphylokinase from Bacillus subtilis. Biotech Bioeng 62:87–96
Demir T, Gübe Ö, Yücel M, Hameş-Kocabaş EE (2013) Increased alkalotolerant and thermostable ribonuclease (RNase) production from alkaliphilic Streptomyces sp. M49–1 by optimizing the growth conditions using response surface methodology. World J Microbiol Biotechnol 29:1625–1633
Park K, Reardon KF (1996) Medium optimization for recombinant protein production by Bacillus subtilis. Biotech Lett 18:737–740
Acknowledgments
This work was supported by the National Science Foundation of China (31071922) and grants from Science and Technology Plan Project of Henan Province (112102310325) and Henan University of Technology (11JCYJ10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no competing financial interests in the findings of this study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Li, RF., Wang, B., Liu, S. et al. Optimization of the Expression Conditions of CGA-N46 in Bacillus subtilis DB1342(p-3N46) by Response Surface Methodology. Interdiscip Sci Comput Life Sci 8, 277–283 (2016). https://doi.org/10.1007/s12539-015-0115-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-015-0115-x