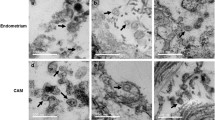
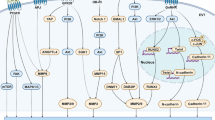
Abstract
Processes of conceptus implantation and placentation, unique to mammalian reproduction, have been extensively studied. It was once thought that processes of these events varied greatly, notably between invasive and noninvasive modes of implantation and/or placentation. Regardless of the mode of implantation, however, physiological and biochemical processes in conceptus implantation to the maternal endometrium including the kinds of gene expression and their products are now considered not to differ so much. Recent progress has identified that in addition to the hormones, cytokines, proteases and cell adhesion molecules classically characterized, epithelial–mesenchymal transition, molecules related to lymphocyte homing, the expression of endogenous retroviruses and possibly exosomes are all required for the progression of conceptus implantation to placentation. In this review, therefore, new findings related to these events are integrated into the context of conceptus implantation to the maternal endometrium.


Similar content being viewed by others
References
Amoroso EC. The evolution of viviparity. J R Soc Med. 1968;61:1188–200.
Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction. 2009;138:195–209.
Hearn JP, Webley GE, Gidley-Baird AA. Chorionic gonadotrophin and embryo-maternal recognition during the peri-implantation period in primates. J Reprod Fertil. 1991;92:497–509.
Soares MJ, Faria TN, Roby KF, Deb S. Pregnancy and the prolactin family of hormones: coordination of anterior pituitary, uterine, and placental expression. Endocr Rev. 1991;12:402–23.
Short RV. Implantation and the maternal recognition of pregnancy. In: Wolstenhome GEW, O’Connor M, editors. “Foetal Anatomy”, Ciba Foundation Symposium. London: J&A Churchill; 1969. p. 2–26.
Quagliarello J, Goldsmith L, Steinetz B, Lustig DS, Weiss G. Induction of relaxin secretion in nonpregnant women by human chorionic gonadotropin. J Clin Endocrinol Metab. 1980;51:74–7.
Godkin JD, Bazer FW, Moffatt J, Sessions F, Roberts RM. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13–21. J Reprod Fertil. 1982;65:141–50.
Imakawa K, Anthony RV, Kazami M, Marotti KR, Polites HG, Roberts RM. Interferon-like sequence of ovine trophoblast protein secreted by embryonic trophectoderm. Nature. 1987;330:377–9.
Stewert HJ, McCann SHE, Barker PJ, Lee KE, Lamming GE, Flint APF. Interferon sequence homology and receptor binding activity of ovine trophoblast antiluteolytic protein. J Endocrinol. 1987;115:R13–5.
Charpigny G, Reinard P, Huet JC, Guillomot M, Charlier M, Pernollet JC, Martal J. High homology between a trophoblastic protein (trophoblastin) isolated from ovine embryo and α-interferon. FEBS Lett. 1988;228:12–6.
Roberts RM, Cross JC, Leaman DW. Interferons as hormones of pregnancy. Endocr Rev. 1992;13:432–52.
Imakawa K, Hansen TR, Malathy PV, Anthony RV, Polites HG, Marotti KR, Roberts RM. Molecular cloning and characterization of complementary deoxyribonucleic acids corresponding to bovine trophoblast-1: a comparison with ovine trophoblast protein-1 and bovine interferon-alpha II. Mol Endocrinol. 1989;3:127–39.
Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annual Rev Biochem. 1987;56:727–77.
Niwano Y, Hansen TR, Kazemi M, Malathy PV, Johnson HD, Roberts RM, Imakawa K. Suppression of T-lymphocyte blastogenesis by ovine trophoblast protein-1 and human interferon-alpha may be independent of interleukin-2 production. Am J Reprod Immunol. 1989;20:21–6.
Roberts RM, Imakawa K, Niwano Y, Kazemi M, Malathy PV, Hansen TR, Glass AA, Kronenberg LH. Interferon production by the preimplantation sheep embryo. J Interferon Res. 1989;9:175–87.
Pontzer CH, Bazer FW, Johnson HM. Antiproliferative activity of a pregnancy recognition hormone, ovine trophoblast protein-1. Cancer Res. 1991;51:5304–7.
Pontzer CH, Yamamoto JK, Bazer FW, Ott TL, Johnson HM. Potent anti-feline immunodeficiency virus and anti-human immunodeficiency virus effect of IFN-τ. J Immunol. 1997;158:4351–7.
Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32.
Rosenfeld CS, Han CS, Alexenko AP, Spencer TE, Roberts RM. Expression of interferon receptor subunits, IFNAR1 and IFNAR2, in the ovine uterus. Biol Reprod. 2002;67:847–53.
Imakawa K, Tamura K, Lee R-S, Ji Y, Kogo H, Sakai S, Christenson RK. Temporal expression of type I interferon receptor in the peri-implantation ovine extra-embryonic membranes: demonstration that human IFN-alpha can bind to this receptor. Endocr J. 2002;49:195–205.
Ott TL, Yin J, Wiley AA, Kim HT, Gerami-Naini B, Spencer TE, Bartol FF, Burghardt RC, Bazer FW. Effects of the estrous cycle and early pregnancy on uterine expression of Mx protein in sheep (Ovis aries). Biol Reprod. 1998;59:784–94.
Johnson GA, Spencer TE, Hansen TR, Austin KJ, Burghardt RC, Bazer FW. Expression of the interferon tau inducible ubiquitin cross-reactive protein in the ovine uterus. Biol Reprod. 1999;61:312–8.
Hicks BA, Etter SJ, Carnahan KG, Joyce MM, Assiri AA, Carling S, Kodali K, Johnson GA, Hansen TR, Mirando MA, Woods GL, Vanderwall DK, Ott TL. Expression of the uterine Mx protein in cyclic and pregnant cows, gilts, and mares. J Anim Sci. 2003;81:1552–62.
Shirasuna K, Nitta A, Sineenard J, Shimizu T, Bollwein H, Miyamoto A. Vascular and immune regulation of corpus luteum development, maintenance, and regression in the cow. Domest Anim Endocrinol. 2012;43:198–211.
Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Ann Rev Biochem. 1998;67:227–64.
Kim S, Choi Y, Bazer FW, Spencer TE. Identification of genes in the ovine endometrium regulated by interferon tau independent of signal transducer and activator of transcription 1. Endocrinology. 2003;144:5203–14.
Chen Y, Antoniou E, Liu Z, Hearne LB, Roberts RM. A microarray analysis for genes regulated by interferon-τ in ovine luminal epithelial cells. Reproduction. 2007;134:123–35.
Spencer TE, Sandra O, Wolf E. Genes involved in conceptus-endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches. Reproduction. 2008;135:165–79.
Gray CA, Adelson DL, Bazer FW, Burghardt RC, Meeusen EN, Spencer TE. Discovery and characterization of an epithelial-specific galectin in the endometrium that forms crystals in the trophectoderm. Proc Natl Acad Sci USA. 2004;101:7982–7.
Mohamed OA, Jonaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/β-catenin signaling is required for implantation. Proc Natl Acad Sci USA. 2005;102:8579–84.
Nagaoka K, Sakai A, Nojima H, Suda Y, Yokomizo Y, Imakawa K, Sakai S, Christenson RK. Expression of a chemokine, IFN-gamma-inducible protein 10 kDa, is stimulated by IFN-τ in the ovine endometrium. Biol Reprod. 2003;68:1413–21.
Imakawa K, Imai M, Sakai A, Suzuki M, Nagaoka K, Sakai S, Lee S-R, Chang K-T, Echterrnkamp SE, Christenson RK. Regulation of conceptus adhesion by endometrial CXC chemokines during the implantation period in sheep. Mol Reprod Dev. 2006;73:850–58.
Nagaoka K, Nojima H, Watanabe F, Christenson RK, Sakai S, Imakawa K. Regulation of blastocyst migration, apposition and initial adhesion by a chemokine, IFN-γ-inducible protein 10 kDa (IP-10), during early gestation. J Biol Chem. 2003;278:29048–56.
Imakawa K, Nagaoka K, Nojima H, Hara Y, Christenson RK. Changes in immune cell distribution and IL-10 production are regulated through endometrial IP-10 expression in the goat uterus. Am J Reprod Immunol. 2005;53:54–64.
Aplin JD, Hey NA, Graham RA. Human endometrial MUC1 carries keratan sulfate: characteristic glycoforms in the luminal epithelium at receptivity. Glycobiology. 1998;8:269–76.
Armant DR. Blastocysts don’t go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells. Dev Biol. 2005;280:260–80.
Akiyama SK. Integrins in cell adhesion and signaling. Hum Cell. 1996;9:181–6.
MacIntyre DM, Lim HC, Ryan K, Kimmins S, Small JA, MacLaren LA. Implantation-associated changes in bovine uterine expression of integrins and extracellular matrix. Biol Reprod. 2002;66:1430–6.
Pfarrer C, Hirsch P, Guillomot M, Leiser R. Interaction of integrin receptors with extracellular matrix is involved in trophoblast giant cell migration in bovine placentomes. Placenta. 2003;24:588–97.
Pfarrer CD. Characterization of the bovine placenta by cytoskeleton, integrin receptors, and extracellular matrix. Methods Mol Med. 2006;121:323–35.
Yamakoshi S, Bai R, Chaen T, Ideta A, Aoyagi Y, Sakurai T, Konno T, Imakawa K. Expression of mesenchymal-related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction. 2012;143:377–87.
Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–11.
May MJ, Entwistle G, Humphries MJ, Ager A. VCAM-1-1 is a CS1 peptide-inhibitable adhesion molecule expressed by lymph node high endothelium. J Cell Sci. 1993;106:109–19.
Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J Gastroenterol. 2003;9:1409–14.
Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. Targeted disruption of the murine VCAM-1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev. 1995;9:1–14.
Konac E, Alp E, Onen HI, Korucuoglu U, Biri AA, Menevse S. Endometrial mRNA expression of matrix metalloproteinases, their tissue inhibitors and cell adhesion molecules in unexplained infertility and implantation failure patients. Reprod Biomed Online. 2009;19:391–7.
Rahman AN, Snibson KJ, Lee CS, Meeusen EN. Effects of implantation and early pregnancy on the expression of cytokines and vascular surface molecules in the sheep endometrium. J Reprod Immunol. 2004;64:45–58.
Henninger DD, Panés J, Eppihimer M, Russell J, Gerritsen M, Anderson DC, Granger DN. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997;158:1825–32.
Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and non-lymphoid sites: getting there and staying there. Crit Rev Immunol. 2009;29:87–109.
Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of β 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Develop. 1995;9:1883–95.
Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development. 1995;121:549–60.
Bai R, Bai H, Kuse M, Ideta A, Aoyagi Y, Fujiwara H, Okuda K, Imakawa K, Sakurai T. Involvement of VCAM1 in the bovine conceptus adhesion to the uterine endometrium. Reproduction. 2014;148:119–27.
Sakurai T, Bai H, Bai R, Arai M, Iwazawa M, Zhang J, Konno T, Godkin JD, Okuda K, Imakawa K. Coculture system that mimics in vivo attachment processes in bovine trophoblast cells. Biol Reprod. 2012;87:1–11.
Biggers JD, Bell JE, Benos DL. Mammalian blastocyst: transport functions in a developing epithelium. Am J Physiol. 1988;255:C419–32.
Kang HM, Kim K, Kwon HB, Cho WK. Regulation of laminin gene expression in the expansion of mouse blastocysts. Mol Reprod Dev. 1990;27:191–9.
Fleming TP, Sheth B, Fesenko I. Cell adhesion in the preimplantation mammalian embryo and its role in trophectodermal differentiation and blastocyst morphogenesis. Front Biosci. 2000;6:1000–7.
Denker HW. Implantation: a cell biological paradox. J Exp Zool. 1993;266:541–58.
Wathes DC, Wooding FB. An electron microscopic study of implantation in the cow. Am J Anat. 1980;159:285–306.
Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype. Nat Rev Cancer. 2007;7:415–28.
Wang X, Zheng M, Liu G, Xia W, McKeown-Longo PJ, Hung MC, Zhao I. Küppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res. 2007;67:7184–93.
Yang J, Weinberg RA. Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Develop Cell. 2008;14:818–29.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelium-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Mess A, Carter AM. Evolutionary transformations of fetal membrane characters in Eutheria with special reference to Afrotheria. J Exp Zool B Mol Dev Evol. 2006;306:140–63.
Mess A. Evolutionary transformations of chorioallantoic placental characters in Rodentia with special reference to hystricognath species. J Exp Zool A Comp Exp Biol. 2003;299:78–98.
Welsh AO, Enders AC. Trophoblast-decidual cell interactions and establishment of maternal blood circulation in the parietal yolk sac placenta of the rat. Anat Rec. 1987;217:203–19.
Enders AC, Blankenship TN, Conley AJ, Jones CJ. Structure of the midterm placenta of the spotted hyena, Crocuta crocuta, with emphasis on the diverse hemophagous regions. Cells Tissues Organs. 2006;183:141–55.
Enders AC, Carter AM. What can comparative studies of placental structure tell us?—a review. Placenta. 2004;25 Suppl A:S3–9.
de Parseval N, Lazar V, Casella JF, Benit L, Heidmann T. Survey of human genes of retroviral origin: identification and transcriptome of the genes with coding capacity for complete envelope proteins. J Virol. 2003;77:10414–22.
Blaise S, de Parseval N, Heidmann T. Functional characterization of two newly identified Human Endogenous Retrovirus coding envelope genes. Retrovirology. 2005;2:19.
Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, Keith JC, McCoy JM. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–9.
Blaise S, de Parseval N, Benit L, Heidmann T. Genomewide screening for fusogenic human endogenous retrovirus envelops identifies syncytins 2, a gene conserved on primate evolution. Proc Natl Acad Sci USA. 2003;100:13013–8.
Be L, Kanellopoulos C, Sapin V, Dupressoir A, Marceau G, Heidmann T. Placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Science. 2005;102:725–30.
Arnaud F, Caporale M, Varela M, Biek R, Chessa B, Alberti A, Golder M, Mura M, Zhang YP, Yu L, Pereira F, Demartini JC, Leymaster K, Spencer TE, Palmarini M. A paradigm for virus-host coevolution: sequential counter-adaptations between endogenous and exogenous retroviruses. PLoS Pathog. 2007;11:e170.
Heidmann O, Vernochet C, Dupressoir A, Heidmannn T. Identification of an endogenous retroviral envelope gene with fusogenic activity and placenta-specific expression in the rabbit: a new “syncytins” in a third order of mammals. Retrovirology. 2009;6:107.
Cornelis G, Heidmann O, Bernard-Stoecklin S, Reynaud K, Véron G, Mulot B, Dupressoir A, Heidmann T. Ancestral capture of syncytin-Car1, a fusogenic endogenous retroviral envelope gene involved in placentation and conserved in Carnivora. Proc Natl Acad Sci USA. 2012;109:E432–41.
Koshi K, Suzuki Y, Nakaya Y, Imai K, Hosoe M, Takahashi T, Kizaki K, Miyazawa T, Hashizume K. Bovine trophoblastic cell differentiation and binucleation involves enhanced endogenous retrovirus element expression. Reprod Biol Endocrinol. 2012;10:41.
Cornelis G, Heidmann O, Degrelle SA, Vernochet C, Lavialle C, Letzelter C, Bernard-Stoecklin S, Hassanin A, Mulot B, Guillomot M, Hue I, Heidmann T, Dupressoir A. Captured retroviral envelope syncytin gene associated with the unique placental structure of higher ruminants. Proc Natl Acad Sci USA. 2013;110:E828–37.
Cornelis G, Vernochet C, Carradec Q, Souquere S, Mulot B, Catzeflis F, Nilsson MA, Menzies BR, Renfree MB, Pierron G, Zeller U, Heidmann O, Dupressoir A, Heidmann T. Retroviral envelope gene captures and syncytin exaptation for placentation in marsupials. Proc Natl Acad Sci USA. 2015;112:E487–96.
Boeke JD, Stoye JP. Retrotransposons, Endogenous Retroviruses, and the Evolution of Retroelements. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997.
Best S, Le Tissier PR, Stoye JP. Endogenous retroviruses and the evolution of resistance to retroviral infection. Trends Microbiol. 1997;5:313–8.
Harris JR. Placental endogenous retrovirus (ERV): structural, functional, and evolutionary significance. BioEssays. 1998;20:307–16.
Rawn SM, Cross JC. The evolution, regulation, and function of placenta-specific genes. Annu Rev Cell Dev Biol. 2008;24:159–81.
Macfarlan TS, Gifford WD, Driscoll S, Lettieri K, Rowe HM, Bonanomi D, Firth, Singer O, Trono D, Pfaff SL. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature. 2012;487:57–63.
Huppertz B, Kaufmann P, Kingdom J. Trophoblast turnover in health and diseases. Fetal Matern Med Rev. 2002;13:103–18.
Dupressoir A, Marceau G, Vernochet C, Bénit L, Kanellopoulos C, Sapin V, Heidmann T. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc Natl Acad Sci USA. 2005;102:725–30.
Schubert SW, Lamoureux N, Kilian K, Klein-Hitpass L, Hashemolhosseini S. Identification of integrin-alpha4, Rb1, and syncytin a as murine placental target genes of the transcription factor GCMa/Gcm1. J Biol Chem. 2008;283:5460–5.
Schreiber J, Riethmacher-Sonnenberg E, Riethmacher D, Tuerk EE, Enderich J, Bösl MR, Wegner M. Placental failure in mice lacking the mammalian homolog of glial cells missing, GCMa. Mol Cell Biol. 2000;20:2466–74.
Mangeney M, Renard M, Schlecht-Louf G, Bouallaga I, Heidmann O, Letzelter C, Richaud A, Ducos B, Heidmann T. Placental syncytins: genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc Natl Acad Sci USA. 2007;104:20534–9.
Wooding FB, Beckers JF. Trinucleate cells and the ultrastructural localization of bovine placental lactogen. Cell Tissue Res. 1987;247:667–73.
Dunlap KA, Palmarini M, Varela M, Burghardt RC, Hayashi K, Farmer JL, Spencer TE. Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc Natl Acad Sci USA. 2006;103:14390–5.
Baba K, Nakaya Y, Shojima T, Muroi Y, Kizaki K, Hashizume K, Imakawa K, Miyazawa T. Identification of novel endogenous betaretroviruses which are transcribed in the bovine placenta. J Virol. 2011;85:1237–45.
Nakaya Y, Koshi K, Nakagawa S, Hashizume K, Miyazawa T. Fematrin-1 is involved in fetomaternal cell-to-cell fusion in Bovinae placenta and has contributed to diversity of ruminant placentation. J Virol. 2013;87:10563–72.
Wooding FB. Current topic: the synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta. 1992;13:101–13.
Carter AM. Evolution of placental structure and function in ruminants. In: Juengel JE, Miyamoto A, Price C, Reynolds LP, Smith MF, Webb R, editors. Reproduction in domestic ruminants VIII. England: Context Products Ltd. 2014. p. 387–99.
Nakagawa S, Bai H, Sakurai T, Nakaya Y, Konno T, Miyazawa T, Gojobori T, Imakawa K. Dynamic evolution of endogenous retrovirus-derived genes expressed in bovine conceptuses during the period of placentation. Genome Biol Evol. 2013;5:296–306.
Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–95.
Rechavi O, Goldstein I, Kloog Y. Intercellular exchange of proteins: the immune cell habit of sharing. FEBS Lett. 2009;583:1792–9.
Mincheva-Nilsson L, Baranov V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. Am J Reprod Immunol. 2014;72:440–57.
Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360.
Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63:520–33.
Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;820:940–8.
Gong R, Peng X, Kang S, Feng H, Huang J, Zhang W, Lin D, Tien P, Xiao G. Structural characterization of the fusion core in syncytin, envelope protein of human endogenous retrovirus family W. Biochem Biophys Res Commun. 2005;331:1193–200.
Record M. Intercellular communication by exosomes in placenta: a possible role in cell fusion? Placenta. 2014;35:297–302.
Laulagnier K, Grand D, Dujardin A, Hamdi S, Vincent-Schneider H, Lankar D, Salles JP, Bonnerot C, Perret B, Record M. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004;572:11–4.
Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–82.
Huppertz B, Borges M. Placenta trophoblast fusion. Methods Mol Biol. 2008;475:135–47.
Record M. Exosomal lipids in cell–cell communication. In: Zhang H-G, editor. Emerging concepts of tumor exosome-mediated cell–cell communication. USA: Springer; 2012. p. 47–68.
Acknowledgments
This was supported by the Program for Promotion of Basic research Activities for Innovative Bioscience (BRAIN) and by Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry. The authors would like to thank Mr. Robert Moriarty for his thorough evaluation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Imakawa, K., Bai, R., Fujiwara, H. et al. Conceptus implantation and placentation: molecules related to epithelial–mesenchymal transition, lymphocyte homing, endogenous retroviruses, and exosomes. Reprod Med Biol 15, 1–11 (2016). https://doi.org/10.1007/s12522-015-0215-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12522-015-0215-7