Abstract
Background
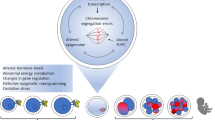
A postovulatory mammalian oocyte decreases developmental potential with in vivo aging in the oviduct or in vitro aging in the culture dish. The mechanism underlying oocyte aging still largely remains an enigma. Accumulating data suggest that the epigenetic alterations such as histone acetylation are also associated with postovulatory aging.
Objective
To perform a review evaluating a new aspect of oocyte aging in terms of the epigenetic alterations focusing on lysine acetylation.
Methods
In addition to a search of the literature in Pubmed, we introduced our recent published data.
Results
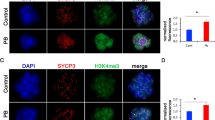
Histone acetylation in the mouse oocyte increases during aging, potentially impacting gene regulation in the subsequent embryonic development. Oocyte aging results in increased acetylation of alpha-tubulin, a non-histone protein, and nicotinamide, an inhibitor of class III HDAC, partially prevents some of oocyte aging phenotypes.
Conclusion
Abnormal regulation of protein acetylation itself is suggested in oocyte aging and could contribute to the aging phenotypes.


Similar content being viewed by others
References
Austin CR. In: Whittaher JR, editor. Concepts of development. Sunderland: Sinauer Associates Inc., Publisher; 1974.
Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:573–85.
Lanman JT. Delays during reproduction and their effects on the embryo and fetus. 2. Aging of eggs. New Engl J Med. 1968;278:1047–54.
Sakai N, Endo A. Effects of delayed mating on preimplantation embryos in spontaneously ovulated mice. Gamete Res. 1988;19:381–5.
Takase K, Ishikawa M, Hoshiai H. Apoptosis in the degeneration process of unfertilized mouse ova. Tohoku J Exp Med. 1995;175:69–76.
Morita Y, Tilly JL. Oocyte apoptosis: like sand through an hourglass. Dev Biol. 1999;213:1–17.
Perez GI, Tao XJ, Tilly JL. Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. Mol Hum Reprod. 1999;5:414–20.
Gordo AC, Rodrigues P, Kurokawa M, Jellerette T, Exley GE, Warner C, Fissore R. Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol Reprod. 2002;66:1828–37.
Liang X, Ma J, Schatten H, Sun Q. Epigenetic changes associated with oocyte aging. Sci China Life Sci. 2012;55:670–6.
Ma JY, Liang XW, Schatten H, Sun QY. Active DNA demethylation in mammalian preimplantation embryos: new insights and new perspectives. Mol Hum Reprod. 2012;18:333–40.
Clayton AL, Hazzalin CA, Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell. 2006;23:289–96.
Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23.
Lee AR, Kishigami S, Amano T, Matsumoto K, Wakayama T, Hosoi Y. Nicotinamide: a class III HDACi delays in vitro aging of mouse oocytes. J Reprod Dev. 2013;59:238–44.
Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci USA. 1964;61:786–94.
Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8:284–95.
Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601.
Huang JC, Yan LY, Lei ZL, Miao YL, Shi LH, Yang JW, Wang Q, Ouyang YC, Sun QY, Chen DY. Change in histone acetylation during postovulatory aging of mouse oocyte. Biol Reprod. 2007;77:666–70.
Liu N, Wu YG, Lan GC, Sui HS, Ge L, Wang JZ, Liu Y, Qiao TW, Tan JH. Pyruvate prevents aging of mouse oocytes. Reproduction. 2009;138:223–34.
Ono T, Mizutani E, Li C, Yamagata K, Wakayama T. Offspring from intracytoplasmic sperm injection of aged mouse oocytes treated with caffeine or MG132. Genesis. 2011;49:460–71.
Cui MS, Wang XL, Tang DW, Zhang J, Liu Y, Zeng SM. Acetylation of H4K12 in porcine oocytes during in vitro aging: potential role of ooplasmic reactive oxygen species. Theriogenology. 2011;75:638–46.
Liang XW, Zhu JQ, Miao YL, Liu JH, Wei L, Lu SS, Hou Y, Schatten H, Lu KH, Sun QY. Loss of methylation imprint of SNRPN in postovulatory aging mouse oocyte. Biochem Biophys Res Commun. 2008;371:16–21.
Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–60.
Wang F, Kou Z, Zhang Y, Gao S. Dynamic reprogramming of histone acetylation and methylation in the first cell cycle of cloned mouse embryos. Biol Reprod. 2007;77:1007–16.
Schatten G, Simerly C, Asai DJ, Szöke E, Cooke P, Schatten H. Acetylated alpha-tubulin in microtubules during mouse fertilization and early development. Dev Biol. 1988;130:74–86.
Matsubara K, Lee AR, Kishigami S, Ito A, Matsumoto K, Chi H, Nishino N, Yoshida M, Hosoi Y. Dynamics and regulation of lysine-acetylation during one-cell stage mouse embryos. Biochem Biophys Res Commun. 2013;434:1–7.
Yoshida N, Brahmajosyula M, Shoji S, Amanai M, Perry AC. Epigenetic discrimination by mouse metaphase II oocytes mediates asymmetric chromatin remodeling independently of meiotic exit. Dev Biol. 2007;301:464–77.
Kawamura Y, Uchijima Y, Horike N, Tonami K, Nishiyama K, Amano T, Asano T, Kurihara Y, Kurihara H. Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J Clin Invest. 2010;120:2817–28.
Akiyama T, Nagata M, Aoki F. Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. Proc Natl Acad Sci USA. 2006;103:7339–44.
Ito M, Sakai S, Nagata M, Aoki F. Effect of histone deacetylases inhibitors on early preimplantation development in mouse embryo. J Mammal Ova Res. 2000;17:90–9.
Ma J, Svoboda P, Schultz RM, Stein P. Regulation of zygotic gene activation in the preimplantation mouse embryo: global activation and repression of gene expression. Biol Reprod. 2001;64:1713–21.
Kishigami S, Ohta H, Mizutani E, Wakayama S, Bui HT, Thuan NV, Hikichi T, Suetsugu R, Wakayama T. Harmful or not: trichostatin A treatment of embryos generated by ICSI or ROSI. Cent Euro J Biol. 2006;1:376–85.
Kishigami S, Mizutani E, Ohta H, Hikichi T, Thuan NV, Wakayama S, Bui HT, Wakayama T. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem Biophys Res Commun. 2006;340:183–9.
Ma P, Schultz RM. Histone deacetylase 2 (HDAC2) regulates chromosome segregation and kinetochore function via H4K16 deacetylation during oocyte maturation in mouse. PLoS Genet. 2013;9:e1003377.
Verdel A, Seigneurin-Berny D, Faure AK, Eddahbi M, Khochbin S, Nonchev S. HDAC6-induced premature chromatin compaction in mouse oocytes and fertilised eggs. Zygote. 2003;11:323–8.
Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol. 2005;283:40–57.
Acknowledgments
We acknowledge discussions with T. Castranio. We are grateful to all the laboratory members for their comments and help. Part of the work cited in this paper was supported by the PRESTO (Precursory Research for Embryonic Science and Technology) program of the Japan Science and Technology Agency and a Grant-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (grant number 23580416, to SK).
Conflict of interest
The author declares that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lee, A.R., Thanh Ha, L., Kishigami, S. et al. Abnormal lysine acetylation with postovulatory oocyte aging. Reprod Med Biol 13, 81–86 (2014). https://doi.org/10.1007/s12522-013-0172-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12522-013-0172-y