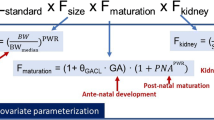
Abstract
Background
Children are in a continuous and dynamically changing state of growth and development. A thorough understanding of developmental pharmacokinetics (PK) and pharmacodynamics (PD) is required to optimize drug therapy in children.
Data sources
Based on recent publications and the experience of our group, we present an outline on integrating pharmacometrics in pediatric clinical practice to develop evidence-based personalized pharmacotherapy.
Results
Antibiotics in septic neonates and immunosuppressants in pediatric transplant recipients are provided as proof-of-concept to demonstrate the utility of pharmacometrics in clinical practice. Dosage individualization based on developmental PK-PD model has potential benefits of improving the efficacy and safety of drug therapy in children.
Conclusion
The pharmacometric technique should be better developed and used in clinical practice to personalize drug therapy in children in order to decrease variability of drug exposure and associated risks of overdose or underdose.
Similar content being viewed by others
References
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 2003;349:1157–1167.
van den Anker JN, Schwab M, Kearns GL. Developmental pharmacokinetics. In: Seyberth HW, Rane A, Schwab M, eds. Pediatric clinical pharmacology. Berlin: Springer, 2011: 51–75.
Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane AR, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ 2000;320:79–82.
Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr 2005;164:552–558.
Conroy S, McIntyre J. The use of unlicensed and off-label medicines in the neonate. Semin Fetal Neonatal Med 2005;10:115–122.
Jacqz-Aigrain E. Drug policy in Europe Research and funding in neonates: Current challenges, future perspectives, new opportunities. Early Hum Dev 2011;87:S27–S30.
Manolis E, Pons G. Proposals for model-based paediatric medicinal development within the current European Union regulatory framework. Br J Clin Pharmacol 2009;68:493–501.
Barrett JS, Fossler MJ, Cadieu KD, Gastonguay MR. Pharmacometrics: A multidisciplinary field to facilitate critical thinking in drug development and translational research settings. J Clin Pharmacol 2008;48:632–649.
Rowland M, Tozer TN. Clinical pharmacokinetics/pharmacodynamics. Philadelphia: Lippincott Williams and Wilkins, 2005.
Mould D, Upton R. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2013;2:e38.
Nielsen EI, Friberg LE. Pharmacokinetic-pharmacodynamic modeling of antibacterial drugs. Pharmacol Rev 2013;65:1053–1090.
Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet 2006;45:683–704.
Zhao W, Fakhoury M, Jacqz-Aigrain E. Developmental pharmacogenetics of immunosuppressants in pediatric organ transplantation. Ther Drug Monit 2010;32:688–699.
Anderson B, Holford N. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008;48:303–332.
Tod M, Jullien V, Pons G. Facilitation of drug evaluation in children by population methods and modelling. Clin Pharmacokinet 2008;47:231–243.
Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci 2013;102:2941–2952.
Anderson BJ, Holford NH. Understanding dosing: children are small adults, neonates are immature children. Arch Dis Child 2013;98:737–744.
Peeters MY, Allegaert K, van Oud-Alblas HJB, Cella M, Tibboel D, Danhof M, et al. Prediction of propofol clearance in children from an allometric model developed in rats, children and adults versus a 0.75 fixed-exponent allometric model. Clin Pharmacokinet 2010;49:269–275.
Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 2009;24:67–76.
De Cock RF, Allegaert K, Schreuder MF, Sherwin CM, de Hoog M, van den Anker JN, et al. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin clearance. Clin Pharmacokinet 2012;51:105–117.
De Cock RF, Allegaert K, Sherwin CM, Nielsen EI, de Hoog M, van den Anker JN, et al. A neonatal amikacin covariate model can be used to predict ontogeny of other drugs eliminated through glomerular filtration in neonates. Pharm Res 2014;31:1–14.
Zhao W, Biran V, Jacqz-Aigrain E. Amikacin maturation model as a marker of renal maturation to predict glomerular filtration rate and vancomycin clearance in neonates. Clin Pharmacokinet 2013;52:1127–1134.
Bellanti F, Della Pasqua O. Modelling and simulation as research tools in paediatric drug development. Eur J Clin Pharmacol 2011;67:75–86.
Barrett JS. Pediatric models in motion: Requirements for modelbased decision support at the bedside. Br J Clin Pharmacol 2013 Nov 20 [Epub ahead of print].
Admiraal R, van Kesteren C, Boelens JJ, Bredius RG, Tibboel D, Knibbe CA. Towards evidence-based dosing regimens in children on the basis of population pharmacokinetic pharmacodynamic modelling. Arch Dis Child 2014;99:267–272.
Manolis E, Osman TE, Herold R, Koenig F, Tomasi P, Vamvakas S, et al. Role of modeling and simulation in pediatric investigation plans. Paediatr Anaesth 2011;21:214–221.
Cohen-Wolkowiez M, Moran C, Benjamin DK, Cotten CM, Clark RH, Benjamin Jr DK, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J 2009;28:1052.
FDA. Guidance for Industry: Exposure-Response Relationships — Study Design, Data Analysis, and Regulatory Applications. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072109.pdf (accessed January 10, 2014).
EMA. Points to consider on Pharmacokinetics and Pharmacodynamics in the development of antibacterial medicinal products (Doc. Ref. CPMP/EWP/2655/99). http://www.ema.europa.eu/docs/enGB/documentlibrary/Scientificguideline/2009/09/WC500003420.pdf (accessed January 10, 2014).
EMA. Note for Guidance on Evaluation of Medicinal Products Indicated for Treatment of Bacterial Infections (CPMP/EWP/558/95 rev 1). http://www.ema.europa.eu/docs/enGB/documentlibrary/Scientificguideline/2009/09/WC500003417.pdf (accessed January 10, 2014).
Polin RA, Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012;129:1006–1015.
Lodise T, Lomaestro B, Drusano G, Society of Infectious Diseases Pharmacists. Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on beta-lactam antibiotics: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2006;26:1320–1332.
Tremoulet A, Le J, Poindexter B, Sullivan JE, Laughon M, Delmore P, et al. Population Pharmacokinetics of Ampicillin in Neonates Using an Opportunistic Study Design. Antimicrob Agents Chemother 2014;58:3013–3020.
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998;26:1–10.
Fisk K. A review of gentamicin use in neonates. Neonatal Netw 1993;12:19–23.
de Cos MA, Gómez-Ullate J, Gómez F, Armijo JA. Time course of trough serum gentamicin concentrations in preterm and term neonates. Clin Pharmacokinet 1992;23:391–401.
Nielsen MEI, Sandström M, Honoré PH, Ewald U, Friberg LE. Developmental pharmacokinetics of gentamicin in preterm and term neonates. Clin Pharmacokinet 2009;48:253–263.
Lowe MN, Lamb HM. Meropenem: an updated review of its use in the management of intra-abdominal infections. Drugs 2000;60:619–646.
Smith PB, Cohen-Wolkowiez M, Castro LM, Poindexter B, Bidegain M, Weitkamp J-H, et al. Population pharmacokinetics of meropenem in plasma and cerebrospinal fluid of infants with suspected or complicated intra-abdominal infections. Pediatr Infect Dis J 2011;30:844.
Jacqz-Aigrain E, Zhao W, Sharland M, van den Anker JN. Use of antibacterial agents in the neonate: 50 years of experience with vancomycin administration. Semin Fetal Neonatal Med 2013;18:28–34.
Dawson PM. Vancomycin and gentamicin in neonates: hindsight, current controversies, and forethought. J Perinat Neonatal Nurs 2002;16:54–72.
Rubin LG, Sánchez PJ, Siegel J, Levine G, Saiman L, Jarvis WR. Evaluation and treatment of neonates with suspected lateonset sepsis: a survey of neonatologists’ practices. Pediatrics 2002;110:e42–e42.
Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet 2012;51:1–13.
Pacifici GM, Allegaert K. Clinical pharmacokinetics of vancomycin in the neonate: a review. Clinics 2012;67:831–837.
de Hoog M, Mouton JW, van den Anker JN. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet 2004;43:417–440.
Zhao W, Lopez E, Biran V, Durrmeyer X, Fakhoury M, Jacqz-Aigrain E. Vancomycin continuous infusion in neonates: dosing optimisation and therapeutic drug monitoring. Arch Dis Child 2013;98:449–453.
de Gatta MdMF, Santos-Buelga D, Domínguez-Gil A, García MJ. Immunosuppressive therapy for paediatric transplant patients. Clin Pharmacokinet 2002;41:115–135.
Cattaneo D, Vinks AA. Optimizing immunosuppressive drug dosing in pediatric renal transplantation: Part of a special series on Paediatric Pharmacology, guest edited by Gianvincenzo Zuccotti, Emilio Clementi, and Massimo Molteni. Pharmacol Res 2012;65:163–167.
Oellerich M, Armstrong VW. The role of therapeutic drug monitoring in individualizing immunosuppressive drug therapy: recent developments. Ther Drug Monit 2006;28:719–725.
Wallemacq P, Armstrong VW, Brunet M, Haufroid V, Holt DW, Johnston A, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit 2009;31:139–152.
Naesens M, Salvatierra O, Li L, Kambham N, Concepcion W, Sarwal M. Maturation of dose-corrected tacrolimus predose trough levels in pediatric kidney allograft recipients. Transplantation 2008;85:1139–1145.
Kausman JY, Patel B, Marks SD. Standard dosing of tacrolimus leads to overexposure in pediatric renal transplantation recipients. Pediatr Transplant 2008;12:329–335.
Hesselink D, Bouamar R, Elens L, Schaik RN, Gelder T. The Role of Pharmacogenetics in the Disposition of and Response to Tacrolimus in Solid Organ Transplantation. Clin Pharmacokinet 2014;53:123–139.
Zhao W, Elie V, Roussey G, Brochard K, Niaudet P, Leroy V, et al. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther 2009;86:609–618.
Zhao W, Fakhoury M, Baudouin V, Storme T, Maisin A, Deschênes G, et al. Population pharmacokinetics and pharmacogenetics of once daily prolonged-release formulation of tacrolimus in pediatric and adolescent kidney transplant recipients. Eur J Clin Pharmacol 2013;69:189–195.
van der Meer AF, Marcus MAE, Touw DJ, Proost JH, Neef C. Optimal Sampling Strategy Development Methodology Using Maximum A Posteriori Bayesian Estimation. Ther Drug Monit 2011;33:133–146.
Zhao W, Fakhoury M, Baudouin V, Maisin A, Deschenes G, Jacqz-Aigrain E. Limited Sampling Strategy for Estimating Individual Exposure of Tacrolimus in Pediatric Kidney Transplant Patients. Ther Drug Monit 2011;33:681–687.
Zhao W, Maisin A, Baudouin V, Fakhoury M, Storme T, Deschenes G, et al. Limited sampling strategy using Bayesian estimation for estimating individual exposure of the once-daily prolonged-release formulation of tacrolimus in kidney transplant children. Eur J Clin Pharmacol 2013;69:1181–1185.
Ettenger R, Sarwal MM. Mycophenolate mofetil in pediatric renal transplantation. Transplantation 2005;80:S201–S210.
Tönshoff B, David-Neto E, Ettenger R, Filler G, van Gelder T, Goebel J, et al. Pediatric aspects of therapeutic drug monitoring of mycophenolic acid in renal transplantation. Transplant Rev 2011;25:78–89.
Barau C, Furlan V, Debray D, Taburet AM, Barrail-Tran A. Population pharmacokinetics of mycophenolic acid and dose optimization with limited sampling strategy in liver transplant children. Br J Clin Pharmacol 2012;74:515–524.
Payen S, Zhang D, Maisin A, Popon M, Bensman A, Bouissou F, et al. Population pharmacokinetics of mycophenolic acid in kidney transplant pediatric and adolescent patients. Ther Drug Monit 2005;27:378–388.
Piana C, Zhao W, Adkison K, Burger D, Jacqz-Aigrain E, Danhof M, et al. Covariate effects and population pharmacokinetics of lamivudine in HIV-infected children. Br J Clin Pharmacol 2013;77:861–872.
Zhao W, Kaguelidou F, Biran V, Zhang D, Allegaert K, Capparelli EV, et al. External evaluation of population pharmacokinetic models of vancomycin in neonates: the transferability of published models to different clinical settings. Br J Clin Pharmacol 2013;75:1068–1080.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, W., Leroux, S. & Jacqz-Aigrain, E. Dosage individualization in children: integration of pharmacometrics in clinical practice. World J Pediatr 10, 197–203 (2014). https://doi.org/10.1007/s12519-014-0493-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-014-0493-x