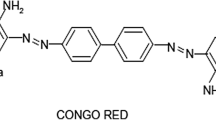
Abstract
From environmental point of view, the removal of effluents from aquatic systems caused by presence of synthetic dyes is extremely important. Oven dried leaf powder solid waste of low-cost bioadsorbent Calotropis procera, has been tested for the removal of azo dye, Congo red (CR) from aqueous solution. Adsorption of CR onto this natural adsorbent has been characterized with X-ray fluorescence, X-ray diffraction, scanning electron microscopy and Fourier transformer infrared. The effects of different parameter such as; contact time, initial dye concentration, adsorbent amount, pH, temperature, electrolyte, surfactant concentration and desorption have been studied. The adsorption has been represented with Langmuir, Freundlich, Tempkin and Dubinin–Radushkevich isotherm models. The maximum adsorption capacity of CR onto bioadsorbent has been found to be 25.77 mg g− 1. The adsorption process has been followed the Weber–Morris Intra-particle diffusion model with the involvement of pseudo second order and Elovich models. The calculated values of thermodynamic parameters such as ΔH and ΔS for uptake of CR have been found to be 35.26 kJ mol− 1 and 120.11 J mol− 1K− 1 respectively. Negative values of ΔG indicate the spontaneous nature of the adsorption process. The results indicate that C. procera has high potential application towards removal of CR dye due to its high adsorption capacity.














Similar content being viewed by others
Abbreviations
- t:
-
Contact time (min)
- T:
-
Temperature (K)
- V:
-
Volume of solution (L)
- W:
-
Mass of dry adsorbent (g)
- C0 :
-
Initial concentration of CR (mg L− 1)
- Ce :
-
Equilibrium concentration (mg L− 1)
- qt :
-
Adsorption capacity of CR at any time t (mg g− 1)
- qe :
-
Adsorption capacity of CR at equilibrium (mg g− 1)
- k1 :
-
Pseudo-first-order rate constant (min− 1)
- k2 :
-
Pseudo-second-order rate constant (g mg− 1 min− 1)
- kip :
-
Intra-particle diffusion rate constant (mg g− 1 min− 1/2)
- C:
-
Thickness of the boundary layer (mg g− 1)
- α:
-
Initial adsorption rate (mg g− 1 min− 1)
- β:
-
The extent of surface coverage (g min− 1)
- qm :
-
Monolayer adsorption capacity (mg g− 1)
- bL :
-
Langmuir constant (L mg− 1)
- RL :
-
Separation factor, dimensionless
- KF :
-
Freundlich constant (mg g− 1)
- 1/n:
-
Heterogeneity factor, dimensionless
- b:
-
Tempkin constant related to heat of adsorption (J mg− 1)
- B:
-
Mean free energy of adsorption (mol2 J− 2)
- qD :
-
Theoretical saturation capacity (mg g− 1)
- R:
-
Universal gas constant (J mol− 1 K− 1)
- R2 :
-
Linear correlation coefficient
- ∆G:
-
Gibbs free energy change (kJ mol− 1)
- ∆H:
-
Enthalpy change (kJ mol-1)
- ∆S:
-
Entropy change (J mol− 1 K− 1)
References
Barka N, Qouzal S, Assabbane A, Nounhan A, Ichou YA (2011) Removal of reactive yellow 84 from aqueous solutions by adsorption onto hydroxyapaite. J Saudi Chem Soc 15:263–267
Barka N, Ouzaouit K, Abdennouri M, El Makhfouk M (2013) Dried prickly pear cactus (Opuntia ficus indica) cladodes as a low-cost and eco-friendly biosorbent for dyes removal from aqueous solutions. J Taiwan Inst Chem Eng 44:52–60
Bohli T, Ouederni A, Fiol N, Villaescusa I (2015) Evaluation of an activated carbon from olive stones used as an adsorbent for heavy metal removal from aqueous phases. C R Chim 18:88–99.
Boudechiche N, Mokaddem H, Sadaoui Z (2016) Biosorption of cationic dye from aqueous solutions onton lignocellulosic biomass (Luffa cylindria): characterization, equilibrium, kinetic and thermodynamic studies. Int J Ind Chem 7:167–180
Carvalho TEM, Fungaro DA, Carina P, Patricia MC (2011) Adsorption of indigo carmine from aqueous solution using coal fly ash and zeolite from fly ash. J Radioanal Nucl Chem 289:617–626
Dahri MK, Kooh MRR, Lim LBL (2015) Application of Casuarina equisetifolia needle for the removal of methylene blue and malachite green dyes from aqueous solution. Alex Eng J 54:1253–1263
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface. Chem Rev 60:235–266
Eddebbagh M, Abourriche A, Berrada M, BenZina M, Bennamara A (2016) Adsorption material from pomegranate (punica granatum) leaves: optimization on removal of methylene blue using response surface methodology. J Mater Environ Sci 7(6):2021–2033
Elahmadi MF, Bensalah N, Gadri A (2009) Treatment of aqueous wastes contaminated with Congo red dye by electrochemical oxidation and ozonation process. J Hazard Mater 168:1163–1169
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57A:385–470
Gupta VK, Mittal A, Gajbe V, Mittal J (2006) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta VK, Carrott PJM, Ribeiro Carrott MML (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Technol 39:783–842
Gupta VK, Rastogi A, Nayak A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342:135–141
Hameed BH, Ahmad AA, Aziz N (2007) Isotherm, kinetics and thermodynamics of acid dye dasorption on activated palm ash. J Chem Eng 1333:195–203
Hameed BH, Tan IAW, Ahmad AL (2008) Optimization of basic dye removal by oil palm fibre-based activated carbon using response surface methodology. J Hazard Mater 158:324–332
Ho YS, Mckay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742
Ho YS, Chiang TH, Hsueh YM (2005) Removal of basic dye from aqueous solution using tree fern as a biosorbent. Process Biochem 40:119–124
Huang L, Zeng G, Huang D, Li L, Huang P, Xia C (2009) Adsorption of lead(II) from aqueous solution onto Hydrilla verticillata. Biodegradation 20:651–660
Iqbal J, Watto FH, Watto MHS, Malik R, Tirmizi SA, Imran M, Ghangro AB (2011) Adsorption of acid yellow dye on flakes of chitosan prepared from fishery waste. Arabian J Chem 4:389–395.
Kaur H, Kaur R (2013) Removal of Rhodamine-B dye from aqueous solution onto pigeon dropping: adsorption, kinetic, equilibrium and thermodynamic studies. J Mater Environ Sci 5(6):1830–1838
Kaur R, Kaur H (2016) Electrochemical degradation of Congo red from aqueous solution: role of graphite anode as electrode material. Port Electrochim Acta 34(3):185–196. doi:10.4152/pea.201603185
Kaur R, Kaur H (2017) Adsorption of amido black 10B from aqueous solution using weed waste as adsorbent: characterization, equilibrium, kinetic and thermodynamic studies. Asian J Chem. doi:10.14233/ajchem.2017.20242
Kaur S, Rani S, Mahajan RK (2013) Adsorption kinetics for the removal of hazardous dye Congo red by biowaste materials as adsorbents. J Chem 2013:1–12
Kaur H, Swati, Kaur R (2014) Kinetic and Isotherm studies of Congo red adsorption from aqueous solution by biowaste material. Chem Sci Trans 3(4):1300–1309. doi:10.7598/cst2014.922
Kaur A, Kaur R, Kaur H (2016) Electrochemical degradation of rhodamine B dye. Mor J Chem 4(1):93–100
Keleşoǧlu S, Kes M, Sütçü L, Polat H (2012) Adsorption of methylene blue from aqueous solution on high lime fly ash: kinetic, equilibrium and thermodynamic studies. J Disper Sci Technol 33:15–23
Khadhhraoui M, Trabelsi H, Bouguerra S, Elleuch B (2008) Discoloration and detoxicification of a Congo red dye solution by means of ozone treatment for a possible water reuse. J Hazard Mater 161:974–981
Kumar PS, Ramalingam S, Senthamarai C, Niranjanaa M, Vijayalakshmi P, Sivanesan S (2010) Adsorption of dye from aqueous solution by cashew nut shell: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 261:52–60
Lachheb H, Puzenat E, Houas A, Ksibi M, Elaloui E, Guillard C, Herrmann JM (2002) Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradation titania. Appl Catal B 39:75–90
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe, Kungliga Sevenska Vetenskapasakademiens. Handlinger 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. doi:10.1021/ja02242a004
Lim LBL, Priyantha N, Tennakoon DTB, Chieng HI, Dahri MK, Suklueng M (2014) Breadnut peel as a highly effective low-cost biosorbent for methylene blue: equilibrium, thermodynamic and kinetic studies. Arabian J Chem. doi: 10.1016/j.arabjc.2013.12.018
Lim LBL, Priyantha N, Chieng HI, Dahri MK (2015) Artocarpus camansi blanco (Breadnet) core as low-cost adsorbent for the removal of methylene blue: equilibrium, thermodynamic and kinetic studies. Desalin Water Treat 7:1–13
Mahmoud DK, Salleh MAM, Karim WAWA, Idris A, Abidin ZZ (2012) Batch adsorption of basic dye using acid treated kenaf fibre char: equilibrium, kinetic and thermodynamic studies. Chem Eng J 181–182:449–457
Meena AK, Yadav A, Rao MM (2011) Ayurvedic uses and pharmacological activities of Calotropis procera Linn. Asian J Tradit Med 6 (2):45–53
Mulinari DR, Voorwald GHJC, Cioffie MOH, Rocha GJ, Da Silva MLCP (2010) Surface modification of sugarcane bagasse cellulose and its effect on mechanical and water absorption properties of sugarcane bagasse cellulose/HDPE composite. Bioresource 5:661–671
Murti Y, Yogi B, Pathak D (2010) Pharmacognostic standardization of leaves of Calotropis procera (Ait.) R. Br. (Asclepiadaceae). Int J Ayurveda Res 1(1):14–17. doi:10.4103/0974-7788.59938
Namasivayam C, Kadirvelu K (1994) Coir pith, an agricultural waste by-product, for the treatment of dyeing wastewater. Bioresour Technol 48:79–81
Namasivayam C, Muniasamy N, Gayatri K, Rani M, Ranganathan K (1996) Removal of dyes from aqueous solutions by cellulosic waste orange peel. Bioresour Technol 57:37–43
Oei BC, Ibrahim S, Wang S, Ang HM (2009) Surfactant modified barley straw for removal of acid and reactive dyes from aqueous solution. Bioresource Technol 100:4292–4295
Patel H, Vashi RT (2012) Removal of Congo red dye from its aqueous solution using natural coagulants. J Saudi Chem Soc 16:131–136
Patil AK, Shrivastava VS (2010) Alternanthera bettzichiana plant powder as low cost adsorbent for removal of Congo red from aqueous solution. Int J Chem Tech Res 2:842–850
Purkait MK, Maiti A, DasGupta S, De S (2007) Removal of Congo red using activated carbon and its regeneration. J Hazard Mater 145:287–295
Reddy MCS (2006) Removal of direct dye from aqueous solution with a novel adsorbent made from tamarind fruit shell, an agricultural solid waste. J Sci Ind Res 65:443–446
Reddy MCS, Sivaramakrishana L, Reddy AV (2012) The use of an agriculture waste material, Jujuba seeds for the removal of anionic dye (Congo red) from aqueous medium. J Hazard Mater 203:118–127.
Sadaf S, Bhatti HN (2014) Batch and fixed bed column studies for the removal of Indosol Yellow BG dye by peanut husk. J Taiwan Inst Chem Eng 45:541–553
Tanobo VOA, Sydenstricker THD, Munaro M, Amico SC (2005) A comprehensive characterization of chemically treated Brazilian sponge-gourds (Luffa cylindria). Polym Test 24:474–482
Tekin N, Bayrak MA, Can E (2016) Adsorption of brilliant yellow onto sepiolite: evaluation of thermodynamics and kinetics and the application of nonlinear isotherm models. J Disper Sci Technol 37:1783–1792
Telke AA, Joshi SM, Jadhav SU, Tamboli DP, Govindwar SP (2010) Decolourization and detoxification of Congo red and textile industry effluent by an isolated bacterium Pseudomonas sp. SU-EBT. Biodegradation 21:283–296
Tor A, Cengeloglu Y (2006) Removal of Congo red from aqueous solution by adsorption onto acid activated red mud. J Hazard Mater 138:409–415
Updegraff DM (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32(3):420–424. doi:10.1016/S0003-2697(69)80009-6
Wang XS, Chen JP (2009) Biosorption of Congo red from aqueous solution using wheat bran and rice bran: batch studies. Sep Sci Technol 44:1452–1466
Wang Y, Shen XY (2012) Optimum plasma surface treatment of luffa fibers. J Macromol Sci Part B Phys 51:662–670
Weber MJ, Morris J (1963) Kinetic of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–59
Acknowledgements
Authors acknowledge sincere thanks to UGC, New Delhi for awarding the UGC-BSR Fellowship (to Ms. Rajvir Kaur) for carrying out the research work successfully.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, R., Kaur, H. Calotropis procera an effective adsorbent for removal of Congo red dye: isotherm and kinetics modelling. Model. Earth Syst. Environ. 3, 9 (2017). https://doi.org/10.1007/s40808-017-0274-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40808-017-0274-3