Abstract
Predicting fluid responsiveness, the response of stroke volume to fluid loading, is a relatively novel concept that aims to optimise circulation, and as such organ perfusion, while avoiding futile and potentially deleterious fluid administrations in critically ill patients. Dynamic parameters have shown to be superior in predicting the response to fluid loading compared with static cardiac filling pressures. However, in routine clinical practice the conditions necessary for dynamic parameters to predict fluid responsiveness are frequently not met. Passive leg raising as a means to alter biventricular preload in combination with subsequent measurement of the change in stroke volume can provide a fast and accurate way to guide fluid management in a broad population of critically ill patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cornerstone of resuscitation of haemodynamically unstable critically ill patients is often considered to be fluid loading. However, only roughly half of haemodynamically unstable patients respond to a fluid challenge, defined as an increase in stroke volume or cardiac output upon fluid loading [1, 2]. Although rapid optimisation of volume status has shown to improve outcome, extended fluid loading is associated with increased morbidity and mortality [3–6]. Little evidence is available for the type and exact dosing of fluid administration. Establishing volume status is complex, making accurate prediction of an increase in stroke volume upon fluid loading, so-called fluid responsiveness, difficult.
Static cardiac filling pressures such as central venous pressure have shown to be incapable of predicting fluid responsiveness accurately. Dynamic parameters on the other hand, using mechanical ventilation-induced changes in preload to track subsequent changes in stroke volume, have shown promise and have been the subject of extensive research in critically ill patients. New methods have been conceived that can easily be used at the bedside in a large variety of patients.
In this review we discuss the basic concepts of fluid responsiveness with a special focus on applications and limitations on use in clinical practice.
Cardiac function and venous return
Since the late 19th century, when Otto Frank demonstrated increased ventricular contraction when the ventricle was stretched prior to contraction, cardiac function has been prominent in our thinking about circulation. Ernest Starling added the knowledge that increasing ventricular filling pressures by increasing venous return led to stroke volume augmentation. These changes in stroke volume in response to changes in venous return and subsequent preload and right atrial pressure (RAP) can be depicted in the well-known Frank-Starling curve. With increasing RAP as a reflection of increased ventricular preload, myocyte stretching increases the sarcomere length with an augmentation in force generation and subsequent increase of stroke volume. The shape of the Frank-Starling curve is dependent on other factors influencing cardiac function besides preload, most notably contractility and afterload. The Frank-Starling curve is, therefore, also known as the cardiac function curve (Fig. 1). The only difference with the classic Frank-Starling curve is that in the cardiac function curve cardiac output is plotted against RAP instead of stroke volume. Therefore the shape of the cardiac function curve is directly affected by heart rate as well.
The cardiac function curve representing the relationship between right atrial pressure (RAP) and cardiac output. The shape of the cardiac function curve will move up with decreased afterload, increased contractility and increased heart rate. Similarly, the curve will move down with increased afterload, decreased contractility and decreased heart rate. Furthermore, when cardiac performance is enhanced, the location of the curve will move leftward by generating a decrease in RAP through a further reduction in the systolic x-wave known from the venous pulse. Similarly, RAP will rise when cardiac performance is decreased and the location of the curve will move to the right. It should be noted that although there is no descending limb illustrated beyond the ‘flat’ part of the curve since actin-myosin myofibrils cannot be disengaged, the secondary effects of increased RAP and preload are not taken into account. For instance, increased preload resulting in ventricular distension with increased wall tension leading to a reduction in coronary perfusion pressure and oxygen delivery could potentially decrease ventricular contractility causing a descending limb in the cardiac function curve
Since the major portion of our blood volume resides in capacitance veins, which are highly distensible, there is a substantial amount of volume not creating transmural pressure, the so-called unstressed volume. The volume that does create a transmural pressure above zero is called the stressed volume determining the mean systemic filling pressure (MSFP). MSFP is the driving force behind venous return, just as mean arterial pressure drives cardiac output. Similarly, venous return is driven by the pressure gradient between the MSFP and RAP limited by the resistance (Rv) venous flow encounters, giving the following equation:
Arthur Guyton has provided useful insight of our understanding of venous return by creating the venous return curve (Fig. 2). As can be deducted from the venous return equation and curve, venous return can be raised by 1) lowering RAP, 2) decreasing Rv, and 3) increasing MSFP.
The venous return curve representing the relationship between right atrial pressure (RAP) and venous return. Baseline venous return curve: RAP becomes equal to mean systemic filling pressure (MSFP) in the absence of flow. Therefore MSFP can be determined at the intercept of the venous return curve with the x-axis. Lowering RAP increases venous return until reaching the critical pressure (Pcrit) at which the great veins at the thoracic inlet start to collapse preventing a further increase in venous return. Increased MSFP: Fluid loading will shift the baseline curve upwards and to the right as MSFP increases more than the rise in RAP with a subsequent increase in venous return. Decreased venous resistance (Rv): Venodilatation will theoretically increase venous return assuming unchanged MSFP, but the expected concomitant decrease in MSFP in practice makes the effect on venous return unpredictable
Means to lower RAP are limited, since the critical pressure (Pcrit) at which the great veins at the thoracic inlet start to collapse causes a subsequent increase in Rv, thereby preventing a further increase in venous return. In the absence of collapsing veins, thus above Pcrit, Guyton confirmed, in extensive experimental models, that venous return changes linearly [7].
Decreasing Rv may be achieved by venodilation. However, by simultaneously increasing venous compliance, MSFP will decrease due to an increase of unstressed volume at the expense of stressed volume. The net effect on venous return is therefore uncertain depending on the relative changes of Rv and MSFP.
Increasing MSFP can be achieved by venoconstriction, but venous return can be limited by an increase in Rv. MSFP can also be increased by enlarging the stressed volume more than RAP by means of fluid loading. Since MSFP is equal to RAP in the absence of circulation, MSFP can be depicted at the horizontal intercept of the venous return curve. Upon fluid loading a right and upward shift is seen with no increase in venous resistance (Fig. 2).
For these reasons, of the three components listed above, venous return can be directly increased by fluid loading. The circulatory system can be described as a composite function curve consisting of input and output of the heart, as venous return and cardiac output have to be equal within a few heartbeats. Subsequently, cardiac output cannot increase without an increase in venous return clarified by the Frank-Starling principle ‘the heart pumps what it receives’. But although increasing preload by fluid loading will increase cardiac output when the patient is on the ascending portion of the cardiac function curve, fluid loading will have little effect when the heart is functioning near the flat part. Guyton made this concept elegantly comprehensible by graphically superimposing the venous return curve on the cardiac function curve, as both are a function of RAP (Fig. 3) [8]. It should be noticed that both curves are in fact a function of preload and thereby represent transmural pressure, i.e. intramural (as RAP is obtained) minus extramural pressure. Therefore a change in extramural intrathoracic pressure can significantly alter preload as has been thoroughly discussed in a recent review [9].
Venous return curves (Fig. 2) superimposed on cardiac function curves (Fig. 1) where the cardiac output and right atrial pressure (RAP) are determined at the junction of the curves assuming a theoretical steady state; in reality, there are fluctuations for instance in RAP with respiration and atrial contractions. Three cardiac function curves are illustrated with different cardiac performances and two venous return curves are depicted with different mean systemic filling pressures (MSFP) obtained by fluid loading. In the curve representing decreased cardiac performance, point I corresponds to a cardiac output which is barely increased by raising MSFP through fluid loading as evidenced by point II signifying fluid unresponsiveness. Point III is reached by increasing cardiac performance not only moving the cardiac function curve upwards resulting in an increase in cardiac output but lowering RAP as well with subsequent increase in venous return without which no increase in cardiac output can be accomplished. Below the critical pressure no increase in cardiac output can be obtained through increasing contractility since no further increase in venous return can be obtained (point IV). Instead cardiac output can be augmented by increasing MSFP (point V)
Cardiac output
Before one aims to increase cardiac output to prevent or treat organ hypoperfusion, it is imperative to first find out whether signs of inadequate tissue perfusion are present. Signs of tissue hypoperfusion for instance are decreased venous oxygen saturation or increased lactate levels, necessitating cardiac output augmentation. Note that subclinical signs of hypoperfusion can easily be missed. When tissue hypoperfusion is likely, it is key to find out a patient’s position on the combined venous return/cardiac function curve to predict whether an increase in cardiac output is to be expected from fluid loading or cardiac function augmentation (Fig. 3). This is especially important since achieving supra-physiological cardiac outputs through inotropics has shown to be detrimental [10]. On the other hand, when decreased cardiac performance with a subsequent rise in RAP and reduced cardiac output is overcome by fluid loading to restore cardiac output, RAP will increase even further. On the ‘flat’ part of the cardiac function curve a rise in MSFP upon fluid loading is accompanied by a similar increase in RAP negating an increase in venous return and cardiac output indicating fluid unresponsiveness (Fig. 3, point I→II). Fluid administration in a non-fluid responsive patient will accelerate a rise in cardiac filling pressures and thus hydrostatic pressures causing pulmonary and general oedema. In that light it is of little surprise that a positive fluid balance has been associated with a worse outcome [11]. Determining volume status is challenging but important since insufficient resuscitation on the other hand has been associated with organ hypoperfusion and ischaemia [12]. The need for predictors of fluid responsiveness is high to select patients who might benefit from fluid loading, and thereby avoiding ineffective and potentially deleterious fluid administration where inotropics may better be used.
Static and dynamic predictors of fluid responsiveness
Central venous pressure as the classic static cardiac filling pressure is still the most often used parameter to guide fluid loading in Dutch critically ill patients [13]. However, endless studies have shown that static cardiac filling pressures are poor predictors of fluid responsiveness [14, 15]. This can largely be explained by three mechanisms. First, cardiac filling pressures are intramural pressures not taking into account extramural pressures and thereby not fully reflecting transmural pressures, which represents preload. This can misguide clinical decision-making when solely using intramurally obtained cardiac filling pressures, especially in mechanically ventilated patients [9]. Furthermore, the combination of cardiac filling pressures with ventricular end-diastolic radius determines preload, which is dependent on ventricular compliance varying largely between patients and within patients when critically ill. Finally, even low cardiac filling pressures do not imply that a patient is fluid responsive (Fig. 3, point I→II). So despite the fact that central venous pressure is a sufficient reflection of RAP as a determinant of right ventricular filling, it is unable to function as a reliable indicator, neither of preload nor of fluid responsiveness. Even right and left ventricular end-diastolic area or volume cannot establish the patient’s position on the combined venous return/cardiac function curve as a decline in cardiac performance decreases the slope of the relationship between end-diastolic volume and stroke volume [16]. Right and left ventricular end-diastolic area and volume can therefore not accurately predict an increase in stroke volume upon fluid loading either [2].
To test fluid responsiveness, a change in preload must be provoked while monitoring the subsequent change in stroke volume or its derivatives such as pulse pressure. In recent years dynamic parameters of fluid responsiveness have been described using mechanical ventilation-induced changes in preload resulting in variation of stroke volume or pulse pressure, so-called stroke volume variation (SVV) and pulse pressure variation (PPV) respectively. Positive pressure mechanical ventilation induces a reduction in left ventricular preload mainly through a decrease in venous return [9]. So when the patient’s position is on the steep portion of the cardiac function curve, mechanical ventilation will induce a larger decrease in stroke volume than when positioned on the flat part of the curve. In other words, SVV induced by mechanical ventilation will be higher when the patient operates on the steep portion of the cardiac function curve predicting an increase in stroke volume upon fluid loading.
SVV can be measured centrally by echocardiography but this method is operator dependent and makes repeated measurement of SVV cumbersome [17]. PPV is obtained directly from the peripheral arterial pressure waveform while SVV can be peripherally derived from subsequent pulse contour analysis of this arterial pressure waveform. Both peripherally derived dynamic parameters are an accurate reflection of central SVV. Indeed, SVV as well as PPV have been found to be far better predictors than static indicators (Table 1) [1, 18–20]. A value above 12% has shown to be highly predictive of fluid responsiveness. Dosing fluids on the basis of these dynamic parameters proved feasible and beneficial [21–23]. Dynamic parameters can also be continuously derived by oesophageal Doppler monitoring or even through non-invasive pulse oximeter plethysmography with retained capability to predict fluid responsiveness [24–26].
However, several limitations in the use of dynamic parameters exist. First, controlled mechanical ventilation must be present to induce the required changes in preload. SVV was found to be inaccurate in patients with spontaneous breathing activity [27]. Furthermore, tidal volumes have to be large enough to facilitate significant changes in preload. SVV is unreliable as a predictor of fluid responsiveness in case of tidal volumes <8 ml/kg [28, 29]. Moreover, a regular heart rhythm has to be present as determination of SVV and PPV becomes highly variable and inaccurate with arrhythmias such as atrial fibrillation. Finally, mechanical ventilation can also induce a decrease in left ventricular preload, primarily through increased RV afterload instead of decreased venous return, especially in view of pulmonary hypertension or RV failure [30, 31]. In that instance a raised SVV does not predict fluid responsiveness and should actually be avoided. This underlines the fact that both the left and right ventricle must function on the ascending portion of the cardiac function curve in order to be fluid responsive. Although it is not possible to monitor continuously, echocardiographic assessment of inferior and superior vena cava distensibility and collapsibility, respectively, have shown to accurately predict fluid responsiveness [32, 33]. Various other methods have been developed, such as the end-expiratory occlusion test, upper arm occlusion pressure and PEEP–induced increase in central venous pressure, to predict fluid responsiveness while avoiding many of the caveats existing for SVV and PPV [34–36].
Clinical use of fluid responsiveness
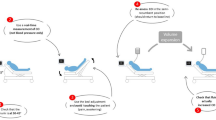
To predict fluid responsiveness, two methods must be combined to generate the changes in preload on one hand and to measure the subsequent changes in stroke volume on the other hand. Both methods must be accurate, fast and easy to use. Since most patients requiring resuscitation are breathing spontaneously, a method other than mechanical ventilation to facilitate changes in preload must be sought. A fluid challenge can be administered, but since only approximately 50% of critically ill patients respond to a fluid challenge, half of patients will receive unnecessary fluid loading. Passive leg raising (PLR) creates a contemporary increase in biventricular preload with a maximum increase within a minute and has shown the capability to predict fluid responsiveness (Fig. 4) [37]. Unsurprisingly, PLR has been demonstrated to be unreliable in case of intra-abdominal hypertension, which impairs venous return [38]. This method is unique in that it has repeatedly shown to be highly predictive when measuring its response on stroke volume even in situations with arrhythmia and spontaneous breathing activity in contrast to SVV and PPV [39, 40]. However, PLR may induce an alteration in vascular compliance by the, potentially painful, postural change reducing its predictive value of fluid responsiveness when solely based on the response in pulse pressure [37]. Measurement of stroke volume can be done before and after PLR by echocardiographic, arterial or oesophageal Doppler to obtain the subsequent changes in stroke volume required for fluid responsiveness prediction without the necessity of a central venous or arterial catheter in place [41–44].
Passive leg raising (PLR) can be performed by elevating the limbs while placing the patient in the supine position to transfer blood both from the lower limbs as from the abdominal compartment creating a sufficient venous return to significantly elevate biventricular preload. Alternatively, classic PLR can be performed by merely elevating the legs with the patient in supine position
However, continuous tracking of stroke volume at the bedside is preferable in clinical practice as it allows evaluation of fluid responsiveness at any time with or without subsequent fluid administrations. With the emergence of several pulse contour analysis methods using the arterial pressure waveforms, stroke volume can be measured continuously without the need for invasive pulmonary artery catheterisation [45–47]. This stroke volume calculation is primarily determined by the pressure decay profile and magnitude of the arterial pressure for a given arterial input impedance. However, very rapid changes in stroke volume occurring during a single breath may not be accurately detected. This can be explained by the possible change in arterial input impedance used to calculate stroke volume [48]. Nevertheless, pulse contour analysis has shown the ability to track the less rapid changes in stroke volume induced by PLR with accurate prediction of fluid responsiveness in patients with spontaneous breathing activity [49]. PLR–induced changes in pulse pressure, as a surrogate for stroke volume, has shown to predict the response to fluid loading as well [50]. Nonetheless, changes in cardiac output upon PLR have been demonstrated to have a better predictive value compared with changes in pulse pressure [39]. This difference can probably be explained by the fact that pulse pressure is not only a direct measure of stroke volume, but also depends on arterial compliance. In other words, the response of arterial pressure upon fluid loading is dependent on arterial tone in contrast to the response of stroke volume. This is important because the aim of haemodynamic resuscitation is not only maintaining adequate organ perfusion by optimising cardiac output, but providing sufficient organ perfusion pressure as well. The arterial pressure response upon fluid loading can be predicted using the PPV to SVV ratio as measure of arterial tone [51]. Since pulse contour analysis has the ability to measure PPV as well as SVV, it is possible to predict ‘blood pressure responsiveness’ besides fluid responsiveness.
Surprisingly, no consensus exists on the exact amount and type of fluid loading nor the timing and cut-off value for cardiac output defining fluid responders. Even the recommended technique of stroke volume measurements or its derivatives such as pulse pressure and cardiac output have not been agreed upon. Although non-invasive measurement would be ideally suited for patients on the ward or for critically ill patients on initial presentation, plethysmographic waveform analysis has shown to be a weak predictor of fluid responsiveness upon PLR in patients with spontaneous breathing activity probably due to acute changes in vasomotor tone [52]. In that respect the emergence of non-invasive bioreactance cardiac output measurements is promising, requiring only four electrodes attached to the body with the ability to predict fluid responsiveness upon PLR [53].
Fluid responsiveness assessment in daily medical practice is feasible when combining an induced change in cardiac preload while measuring the subsequent change in stroke volume or its derivatives such as pulse pressure on the other hand. In this way, one can dynamically elucidate the position of the patient on the combined venous return/cardiac function curve (Fig. 3) and predict the response to fluid loading. It is important to note that a rise in the delivery of oxygen to the tissues to prevent or treat organ hypoperfusion can be obtained through optimisation of the concentration and saturation of haemoglobin besides an increase in cardiac output. Even in the presence of increased delivery of oxygen, impaired oxygen extraction and consumption can be predominant, especially during sepsis. Furthermore, cardiac output is not only determined by preload and contractility, but in particular by afterload and heart rate as well, making the increase in cardiac output by fluid loading just part of the solution. Echocardiography remains advocated in unexplained haemodynamic instability to provide important information regarding contractility and loading conditions of the heart. Finally, trials outside the operating theatre with hard clinical endpoints on morbidity and mortality are needed to validate and stimulate the assessment of fluid responsiveness in critically ill patients.
Conclusion
‘To fill or not to fill’, that is the question frequently faced by physicians caring for haemodynamically unstable critically ill patients. To successfully predict fluid responsiveness, the response of stroke volume to fluid loading, two requirements must be met: on one hand a change in preload must be generated as well as measuring the subsequent changes in stroke volume or its derivatives such as pulse pressure on the other hand. Static markers of cardiac preload are therefore unable to predict fluid responsiveness with dynamic markers being superior. However, a regular heart rhythm and mechanical ventilation without spontaneous breathing activity with larger than currently recommended tidal volumes are necessary for dynamic parameters to predict fluid responsiveness limiting its clinical application. Passive leg raising is an easy alternative tool that can be applied at the bedside to effectively change preload temporarily. Increases in cardiac output by passive leg raising predict fluid responsiveness despite spontaneous breathing activity or cardiac arrhythmias. When used in combination with a direct measure of continuously tracking stroke volume and/or its derivatives, physicians are enabled to prevent and treat organ hypoperfusion at any time, while avoiding unnecessary and potentially harmful fluid loading and inotropics. Understanding the basic concepts of the connection between venous return and cardiac function is pivotal to accomplish tailor-made fluid titration in the treatment of haemodynamically unstable critically ill patients.
References
Marik PE, Cavalazzi R, Vasu T, et al. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642–7.
Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–8.
Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Holte K, Kehlet H. Fluid therapy and surgical outcomes in elective surgery: a need for reassessment in fast-track surgery. J Am Coll Surg. 2006;202:971–89.
Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75.
Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–65.
Guyton AC, Lindsey AW, Abernathy B, et al. Mechanism of the increased venous return and cardiac output caused by epinephrine. Am J Physiol. 1958;192:126–30.
Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev. 1955;35:123–9.
Cherpanath TG, Lagrand WK, Schultz MJ, et al. Cardiopulmonary interactions during mechanical ventilation in critically ill patient. Neth Heart J. 2013;21:166–72.
Hayes MA, Timmins AC, Yau EH, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–22.
Sakr Y, Vincent JL, Reinhart K, et al. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest. 2005;128:3098–108.
Murakawa K, Kobayashi A. Effects of vasopressors on renal tissue gas tensions during hemorrhagic shock in dogs. Crit Care Med. 1988;16:789–92.
Geerts BF, Maas JJ, de Wilde RBP, et al. Hemodynamic assessment in the Dutch intensive care unit. Neth J Crit Care. 2009;13:178–84.
Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–8.
Magder S. Fluid status and fluid responsiveness. Curr Opin Crit Care. 2010;16:289–96.
Braunwald E, Sonnenblick EH, Ross J. Mechanisms of cardiac contraction and relaxation. In: Braunwald E, editor. Heart disease. Philadelphia: Saunders; 1998. p. 389–425.
Feissel M, Michard F, Mangin I, et al. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119:867–73.
Marx G, Cope T, McCrossan L, et al. Assessing fluid responsiveness by stroke volume variation in mechanically ventilated patients with severe sepsis. Eur J Anaesthesiol. 2004;21:132–8.
Reuter DA, Kirchner A, Felbinger TW, et al. Usefulness of left ventricular stroke volume variations to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med. 2003;31:1399–404.
Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103:419–28.
Lopes MR, Oliveira MA, Pereira VO, et al. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care. 2007;11:R100.
Sakka SG, Becher L, Kozieras J, et al. Effects of changes in blood pressure and airway pressures on parameters of fluid responsiveness. Eur J Anaesthesiol. 2009;26:322–7.
Jhanji S, Vivian-Smith A, Lucena-Amaro S, et al. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Crit Care. 2010;14:R151.
Desebbe O, Cannesson M. Using ventilation-induced plethysmographic variations to optimize patient fluid status. Curr Opin Anaesthesiol. 2008;21:772–8.
Feissel M, Teboul JL, Merlani P, et al. Plethysmographic dynamic indices predict fluid responsiveness in septic ventilated patients. Intensive Care Med. 2007;33:993–9.
Monnet X, Rienzo M, Osman D, et al. Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med. 2005;31:1195–201.
Perner A, Faber T. Stroke volume variation does not predict fluid responsiveness in patients with septic shock on pressure support ventilation. Acta Anaesthesiol Scand. 2006;50:1068–73.
De Backer D, Heenen S, Piagnerelli M, et al. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31:517–23.
Reuter DA, Bayerlein J, Goepfert MS, et al. Influence of tidal volume on left ventricular stroke volume variation measured by pulse contour analysis in mechanically ventilated patients. Intensive Care Med. 2003;29:476–80.
Wyler von Ballmoos M, Takala J, Roeck M, et al. Pulse-pressure variation and hemodynamic response in patients with elevated pulmonary artery pressure: a clinical study. Crit Care. 2010;14:R111.
Mahjoub Y, Pila C, Friggeri A, et al. Assessing fluid responsiveness in critically ill patients: false-positive pulse pressure variation is detected by Doppler echocardiographic evaluation of the right ventricle. Crit Care Med. 2009;37:2570–5.
Feissel M, Michard F, Faller JP, et al. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30:1834–7.
Vieillard-Baron A, Chergui K, Rabiller A, et al. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004;30:1734–9.
Monnet X, Osman D, Ridel C, et al. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med. 2009;37:951–6.
Geerts BF, Maas J, de Wilde RB, et al. Arm occlusion pressure is a useful predictor of an increase in cardiac output after fluid loading following cardiac surgery. Eur J Anaesthesiol. 2011;28:802–6.
Geerts BF, Aarts LP, Groeneveld AB, et al. Predicting cardiac output responses to passive leg raising by a PEEP-induced increase in central venous pressure, in cardiac surgery patients. Br J Anaesth. 2011;107:150–6.
Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402–7.
Mahjoub Y, Touzeau J, Airapetian N, et al. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med. 2010;38:1824–9.
Cavallaro F, Sandroni C, Marano C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med. 2010;36:1475–83.
Monnet X, Teboul J. Passive leg raising. Intensive Care Med. 2008;34:659–63.
Lamia B, Ochagavia A, Monnet X, et al. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33:1125–32.
Thiel SW, Kollef MH, Isakow W. Non-invasive stroke volume measurement and passive leg raising predict volume responsiveness in medical ICU patients: an observational cohort study. Crit Care. 2009;13:R111.
Preau S, Saulnier F, Dewavrin F, et al. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010;38:819–25.
Lafanechere A, Pene F, Goulenok C, et al. Changes in aortic blood flow induced by passive leg raising predict fluid responsiveness in critically ill patients. Crit Care. 2006;10:R132.
Hofer CK, Senn A, Weibel I, et al. Assessment of stroke volume variation for prediction of fluid responsiveness using the modified FloTrac and PiCCOplus system. Crit Care. 2008;12:R82.
De Castro V, Goarin JP, Lhotel L, et al. Comparison of stroke volume (SV) and stroke volume respiratory variation (SVV) measured by the axillary artery pulse-contour method and by aortic Doppler echocardiography in patients undergoing aortic surgery. Br J Anaesth. 2006;97:605–10.
De Wilde RB, Schreuder JJ, van den Berg PC, et al. An evaluation of cardiac output by five arterial pulse contour techniques during cardiac surgery. Anaesthesia. 2007;62:760–8.
Pinsky MR. Probing the limits of arterial pulse contour analysis to predict preload responsiveness. Anesth Analg. 2003;96:1245–7.
Biais M, Vidil L, Sarrabay P, et al. Changes in stroke volume induced by passive leg raising in spontaneously breathing patients: comparison between echocardiography and Vigileo/FloTrac device. Crit Care. 2009;13:R195.
Boulain T, Achard JM, Teboul JL, et al. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest. 2002;121:1245–52.
Monge García MI, Gil Cano A, Gracia Romero M. Dynamic arterial elastance to predict arterial pressure response to volume loading in preload-dependent patients. Crit Care. 2011;15:R15.
Keller G, Cassar E, Desebbe O, et al. Ability of pleth variability index to detect hemodynamic changes induced by passive leg raising in spontaneously breathing volunteers. Crit Care. 2008;12:R37.
Benomar B, Ouattara A, Estagnasie P, et al. Fluid responsiveness predicted by noninvasive bioreactance-based passive leg raise test. Intensive Care Med. 2010;36:1875–81.
Acknowledgments
We would like to thank Carolien Paes for her assistance with the figure preparations.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Funding
None.
Conflict of interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Cherpanath, T.G.V., Geerts, B.F., Lagrand, W.K. et al. Basic concepts of fluid responsiveness. Neth Heart J 21, 530–536 (2013). https://doi.org/10.1007/s12471-013-0487-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12471-013-0487-7