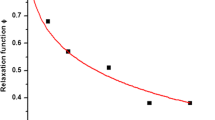
Abstract
The glass transition and corresponding glass transition temperature (T g ) as found for food solids have received considerable attention, and their relationships with food solid behavior in various processes and food storage have been disputed. However, understanding glass transition-associated relaxations and their coupling with physicochemical properties of food materials is still a challenging and developing area of food material science. This review shows the improved understanding of the glass transition, structural relaxations, and corresponding relaxation times (τ) as well as their relevant applications on glassy food solids. Above information contributes to understanding of physical changes, molecular mobility, and solid flow characteristics of food components around their glass transition during processing and storage. We also emphasized the limitations of “fragile” concept in food solids and introduced a “strength, S” parameter to measure and describe solid flow characteristics of amorphous food mixtures by using a William-Landel-Ferry (WLF)-based approach for the analysis of α-relaxation derived τ above T g . The use of the S concept, therefore, can provide a quantitative measure to estimate compositional effects on τ and the control of solid properties, such as stickiness, caking, collapse, and crystallization.







Similar content being viewed by others
References
Acevedo NC, Schebor C, Buera P (2008) Non-enzymatic browning kinetics analysed through water–solids interactions and water mobility in dehydrated potato. Food Chem 108(3):900–906
Adam G, Gibbs JH (1965) On the temperature dependence of cooperative relaxation properties in glass-forming liquids. J Chem Phys 43(1):139–146
Al-Muhtaseb AH, McMinn WAM, Magee TRA (2002) Moisture sorption isotherm characteristics of food products: A review. Food Bioprod Process 80(2):118–128
Anderson SL, Grulke EA, DeLassus PT, Smith PB, Kocher CW, Landes BG (1995) A model for antiplasticization in polystyrene. Macromolecules 28(8):2944–2954
Angell CA (1988) Structural instability and relaxation in liquid and glassy phases near the fragile liquid limit. J Non-Cryst Solids 102(1–3):205–221
Angell CA (1991) Relaxation in liquids, polymers and plastic crystals-strong/fragile patterns and problems. J Non-Cryst Solids 131-133:13–31
Angell CA (2002) Liquid fragility and the glass transition in water and aqueous solutions. Chem Rev 102(8):2627–2650
Angell CA, Poole PH, Shao J (1994) Glass-forming liquids, anomalous liquids, and polyamorphism in liquids and biopolymers. II Nuovo Cimento D 16(8):993–1025
Anglea SA, Wang J, Karel M (1993) Quality changes of eggplant due to drying regime. Presented at Annu meet Inst food Technol Chicago pap: 192.
Balasubramanian S, Devi A, Singh KK, Bosco SD, Mohite AM (2016) Application of glass transition in food processing. Crit Rev Food Sci Nutr 56(6):919–936
Batzer H, Kreibich UT (1981) Influence of water on thermal transitions in natural polymers and synthetic polyamides. Polym Bull 5(11–12):585–590
Borde B, Bizot H, Vigier G, Buleon A (2002) Calorimetric analysis of the structural relaxation in partially hydrated amorphous polysaccharides I. Glass Transit Fragility. Carbohydr Polym 48:83–96
Burin L, Jouppila K, Roos YH, Kansikas J, Buera MP (2004) Retention of β-galactosidase activity as related to Maillard reaction, lactose crystallization, collapse and glass transition in low moisture whey systems. Int Dairy J 14(6):517–525
Cao N, Yang X, Fu Y (2009) Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocolloid 23(3):729–735
Champion D, Le Meste M, Simatos D (2000) Towards an improved understanding of glass transition and relaxations in foods: Molecular mobility in the glass transition range. Trends Food Sci Tech 11(2):41–55
Chan HK, Clark AR, Feeley JC, Kuo MC, Lehrman SR, Pikal-Cleland K, Miller DP, Vehring R, Lechuga-Ballesteros D (2004) Physical stability of salmon calcitonin spray-dried powders for inhalation. J Pharmac Sci 93(3):792–804
Chang YP, Cheah PB, Seow CC (2000) Plasticizing-Antiplasticizing effects of water on physical properties of tapioca starch films in the glassy state. J Food Sci 65(3):445–451
Chuang L, Panyoyai N, Shanks R, Kasapis S (2015) Effect of sodium chloride on the glass transition of condensed starch systems. Food Chem 184:65–71
Cocero AM, Kokini JL (1991) The study of the glass transition of glutenin using small amplitude oscillatory rheological measurements and differential scanning calorimetry. J Rheol 35(2):257–270
Cohen MH, Turnbull D (1959) Molecular transport in liquids and glasses. J Chem Phys 31(5):1164–1169
Crowley KJ, Zografi G (2001) The use of thermal methods for predicting glass former fragility. Thermochim Acta 380(2):79–93
Debenedetti PG, Stillinger FH (2001) Supercooled liquids and the glass transition. Nature 410(6825):259–267
Descamps N, Palzer S, Roos YH, Fitzpatrick JJ (2013) Glass transition and flowability/caking behaviour of maltodextrin DE 21. J Food Eng 119(4):809–813
Donth EJ (2013) The glass transition: Relaxation dynamics in liquids and disordered materials. Springer Science & Business Media.
Duckworth RB (1981) Solute mobility in relation to water content and water activity. In: Rockland, L.B., Stewart, G.F. (Eds.), Water Activity: Influences on Food Quality. Academic Press, New York, NY, pp. 295 317.
Enrione JI, Díaz PC, Matiacevich SB, Hill SE (2012) Mechanical and structural stability of an extruded starch-protein-polyol food system. J Food Res 1(2):224
Eyring H (1936) Viscosity, plasticity, and diffusion as examples of absolute reaction rates. J Chem Phys 4(4):283–291
Fan F, Mou T, Nurhadi B, Roos YH (2017) Water sorption-induced crystallization, structural relaxations and strength analysis of relaxation times in amorphous lactose/whey protein systems. J Food Eng 196:150–158
Fan F, Roos YH (2015) X-ray diffraction analysis of lactose crystallization in freeze-dried lactose–whey protein systems. Food Res Int 67:1–11
Fan F, Roos YH (2016a) Structural relaxations of amorphous lactose and lactose-whey protein mixtures. J Food Eng 173:106–115
Fan F, Roos YH (2016b) Crystallization and structural relaxation times in structural strength analysis of amorphous sugar/whey protein systems. Food. Hydrocolloid. 60:85–97
Fan F, Roos YH (2016c) Structural strength and crystallization of amorphous lactose in food model solids at various water activities. Innov Food Sci Emerg Technol 40:27–34
Ferry JD (1980) Viscoelastic properties of polymers. John Wiley & Sons.
Fox TG Jr, Flory PJ (1950) Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J Appl Phys 21(6):581–591
George P, Lundin L, Kasapis S (2014) Effect of thermal denaturation on the mechanical glass transition temperature of globular protein/co-solute systems. Food Hydrocolloid 41:156–163
Gibbs JH, DiMarzio EA (1958) Nature of the glass transition and the glassy state. J Chem Phys 28(3):373–383
Gibbs JH, DiMarzio EA (1959) Statistical mechanics of helix-coil transitions in biological macromolecules. J Chem Phys 30(1):271–282
Gordon M, Taylor JS (1952) Ideal copolymers and the second-order transitions of synthetic rubbers. I Non-crystalline copolymers. J Appl Phys 2(9):493–500
Hallett J (1963) The temperature dependence of the viscosity of supercooled water. Proc Phys Soc 82:1046–1050
Hancock BC, Shamblin SL, Zografi G (1995) Molecular mobility of amorphous pharmaceutical solids below their glass transition temperatures. Pharm Res 12(6):799–806
Haque MK, Kawai K, Suzuki T (2006) Glass transition and enthalpy relaxation of amorphous lactose glass. Carbohydr Res 341(11):1884–1889
Harris M, Peleg M (1996) Patterns of textural changes in brittle cellular cereal foods caused by moisture sorption. Cereal Chem 73(2):225–231
Hartel RW (2001) Crystallization in foods. Aspen Publishers.
Hatley RH, Mant A (1993) Determination of the unfrozen water content of maximally freeze-concentrated carbohydrate solutions. Int J Bio Macromolec 15(4):227–232
Hodge IM (1996) Strong and fragile liquids-a brief critique. J Non-Cryst Solids 202:164–172
Icoz DZ, Kokini JL (2008) State diagrams of food materials. Food Materials Science pp: 95–121. Springer New York.
Johari GP (1976) Glass transition and secondary relaxations in molecular liquids and crystals. Ann N Y Acad Sci 279(1):117–140
Kaletunc G, Breslauer KJ (1993) Glass transitions of extrudates: Relationship with processing-induced fragmentation and end-product attributes. Cereal Chem 70:548–548
Kalichevsky MT, Blanshard JMV (1992) A study of the effect of water on the glass transition of 1: 1 mixtures of amylopectin, casein and gluten using DSC and DMTA. Carbohydr Polym 19(4):271–278
Kalichevsky MT, Jaroszkiewicz EM, Ablett S, Blanshard JMV, Lillford PJ (1992) The glass transition of amylopectin measured by DSC, DMTA and NMR. Carbohydrate Polymers, 18(2):77–88
Karathanos V (1993) Collapse of structure during drying of celery. Dry Technol 11(5):1005–1023
Karel M (1985) Effects of water activity and water content on mobility in food components, and their effect on phase transitions in food systems. In: Simatos, D., Multon, J. L. (Eds.), Properties of Water in Foods. Martinus Nijhoff publishers, Dordrecht, the Netherlands, pp. 153-169.
Kilburn D, Claude J, Mezzenga R, Dlubek G, Alam A, Ubbink J (2004) Water in glassy carbohydrates: Opening it up at the nanolevel. J Phys Chem B 108(33):12436–12441
Kim YJ, Hagiwara T, Kawai K, Suzuki T, Takai R (2003) Kinetic process of enthalpy relaxation of glassy starch and effect of physical aging upon its water vapor permeability property. Carbohydr Polym 53(3):289–296
Kweon M, Slade L, Levine H (2009) Oxidative gelation of solvent-accessible arabinoxylans is the predominant consequence of extensive chlorination of soft wheat flour. Cereal Chem 86(4):421–424
Le Meste M, Champion D, Roudaut G, Blond G, Simatos D (2002) Glass transition and food technology: A critical appraisal. J Food Sci 67(7):2444–2458
Levi G, Karel M (1995) Volumetric shrinkage (collapse) in freeze-dried carbohydrates above their glass transition temperature. Food Res Int 28(2):145–151
Levine H, Slade L (1989) A food polymer science approach to the practice of cryostabilization technology. Comments Agric Food Chem 1(6):315–396
Lievonen SM, Laaksonen TJ, Roos YH (1998) Glass transition and reaction rates: Nonenzymatic browning in glassy and liquid systems. J Agric Food Chem 46(7):2778–2784
Liu Y, Bhandari B, Zhou W (2006) Glass transition and enthalpy relaxation of amorphous food saccharides: A review. J Agric Food Chem 54(16):5701–5717
Luk E, Sandoval AJ, Cova A, Müller AJ (2013) Anti-plasticization of cassava starch by complexing fatty acids. Carbohydr Polym 98(1):659–664
Maidannyk VA, Roos YH (2016) Modification of the WLF model for characterization of the relaxation time-temperature relationship in trehalose-whey protein isolates systems. J Food Eng 188:21–31
Meister E, Gieseler H (2009) Freeze-dry microscopy of protein/sugar mixtures: Drying behavior, interpretation of collapse temperatures and a comparison to corresponding glass transition data. J Pharm Sci 98(9):3072–3087
Mullin JW (2001) Crystallization. Butterworth-Heinemann
Noel TR, Ring SG, Whittam MA (1993) Relaxations in supercooled carbohydrate liquids. In: Blanshard JMV, Lillford PJ (eds) The glassy state in foods. Nottingham University Press, Loughborough, pp 173–187
Nurhadi B, Roos YH, Maidannyk V (2016) Physical properties of maltodextrin DE 10: Water sorption, water plasticization and enthalpy relaxation. J Food Eng 174:68–74
Parks GS, Gilkey WA (1929) Studies on glass IV. Some viscosity data on liquid glucose and glucose-glycerol solutions. J Phys Chem 33:1428–1437
Paudel A, Raijada D, Rantanen J (2015) Raman spectroscopy in pharmaceutical product design. Adv Drug Deliv Rev 89:3–20
Peleg M (1995) Description of mechanical changes in foods at their glass transition region. In: Food preservations by moisture control: Fundamentals and applications. Technomic Publishing, Lancaster, pp 659–671
Perez J (1994) Theories of liquid-glass transition. J Food Eng 22:89–114
Pittia P, Sacchetti G (2008) Antiplasticization effect of water in amorphous foods. A review. Food Chem 106(4):1417–1427
Porter D, Vollrath F (2012) Water mobility, denaturation and the glass transition in proteins. Biochimi Biophys Acta Rev 1824(6):785–791
Potes N, Kerry JP, Roos YH (2012) Additivity of water sorption, alpha-relaxations and crystallization inhibition in lactose–maltodextrin systems. Carbohydr Polym 89(4):1050–1059
Roos YH (2010) Glass transition temperature and its relevance in food processing. Ann Rev Food Sci Technol 1:469–496
Roos YH (2013a) Relaxations, glass transition and engineering properties of food solids. In: Advances in food process engineering research and applications. Springer, New York, pp 79–90
Roos YH (2013b) Relaxations, glass transition and engineering properties of food solids. In Advances in Food Process Engineering Research and Applications pp: 79–90. Springer US.
Roos YH, Drusch S (2015) Phase transitions in foods. Academic Press
Roos YH, Fryer PJ, Knorr D, Schuchmann HP, Schroën K, Schutyser MAI, Trystram G, Windhab EJ (2016) Food engineering at multiple scales: Case studies, challenges and the future-a European perspective. Food Eng Rev 8(91):91–115
Roos YH, Karel M (1991) Water and molecular weight effects on glass transitions in amorphous carbohydrates and carbohydrate solutions. J Food Sci 56(6):1676–1681
Roos YH, Potes N (2015) Quantification of protein hydration, glass transitions, and structural relaxations of aqueous protein and carbohydrate–protein systems. J Physic Chem B 119(23):7077–7086
Roos YH, Roininen K, Jouppila K, Tuorila H (1998) Glass transition and water plasticization effects on crispness of a snack food extrudate. Int J Food Prop 1:163–180
Roudaut G, Simatos D, Champion D, Contreras-Lopez E, Le Meste M (2004) Molecular mobility around the glass transition temperature: A mini review. Innov. Food. Sci. & Emerg. Technol. 5(2):127–134
Ruiz-Cabrera MA, Schmidt SJ (2015) Determination of glass transition temperatures during cooling and heating of low-moisture amorphous sugar mixtures. J Food Eng 146:36–43
Sasaki K, Matsui Y, Miyara M, Kita R, Shinyashiki N, Yagihara S (2016) Glass transition and dynamics of the polymer and water in the poly (vinylpyrrolidone)–water mixtures studied by dielectric relaxation spectroscopy. J Physic Chem B 120(27):6882–6889
Segur JB, Oberstar HE (1951) Viscosity of glycerol and its aqueous solutions. Int Eng Chem 43(9):2117–2120
Shamblin SL, Zografi G (1998) Enthalpy relaxation in binary amorphous mixtures containing sucrose. Pharm Res 15(12):1828–1834
Shamblin SL, Taylor LS, Zografi G (1998). Mixing behavior of colyophilized binary systems. Journal of pharmaceutical sciences, 87(6):694–701
Shaw NB, Monahan FJ, O’Riordan ED, O’sullivan M (2002) Physical properties of WPI films plasticized with glycerol, xylitol, or sorbitol. J Food Sci 67(1):164–167
Shogren RL (1992) Effect of moisture content on the melting and subsequent physical aging of cornstarch. Carbohydr Polym 19(2):83–90
Singh J, Kaur L, McCarthy OJ (2007) Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications: A review. Food. Hydrocolloid. 21(1):1–22
Sjögren L, Götze W (1994) α-relaxation spectra in supercooled liquids. J Non-Cryst Solids 172:7–15
Slade L, Levine H (1995) Water and the glass transition—Dependence of the glass transition on composition and chemical structure: Special implications for flour functionality in cookie baking. J Food Eng 24(4):431–509
Slade L, Levine H, Reid DS (1991) Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. Crit Rev Food Sci Nutr 30(2–3):115–360
Sothornvit R, Krochta JM (2000) Plasticizer effect on oxygen permeability of β-lactoglobulin films. J Agric Food Chem 48(12):6298–6302
Sperling LH (2005) Introduction to physical polymer science. John Wiley & Sons
Suyatma NE, Tighzert L, Copinet A, Coma V (2005) Effects of hydrophilic plasticizers on mechanical, thermal, and surface properties of chitosan films. J Agric Food Chem 53(10):3950–3957
Syamaladevi RM, Barbosa-Cánovas GV, Schmidt SJ, Sablani SS (2012) Influence of molecular weight on enthalpy relaxation and fragility of amorphous carbohydrates. Carbohydr Polym 88(1):223–231
Tant MR, Wilkes GL (1981) Physical aging studies of semicrystalline poly (ethylene terephthalate). J Appl Polym Sci 26(9):2813–2825
Tarjus G, Kivelson D (1995) Breakdown of the stokes–Einstein relation in supercooled liquids. J Chem Phys 103(8):3071–3073
Telis VRN, Martínez-Navarrete N (2009) Collapse and color changes in grapefruit juice powder as affected by water activity, glass transition, and addition of carbohydrate polymers. Food Biophys 4(2):83–93
Truong V, Bhandari BR, Howes T, Adhikari B (2002) Analytical model for the prediction of glass transition temperature of food systems. In Amorphous Food and Pharmaceutical Systems. The Royal Society of Chemistry, Cambridge pp: 31–47.
Velikov V, Borick S, Angell CA (2001) The glass transition of water, based on hyperquenching experiments. Science 294(5550):2335–2338
Weng L, Elliott GD (2014) Dynamic and thermodynamic characteristics associated with the glass transition of amorphous trehalose–water mixtures. Physic Chem Phys 16(23):11555–11565
Williams ML, Landel RF, Ferry JD (1955) The temperature dependence of relaxation mechanisms in amorphous polymers and other glass-forming liquids. J Am Chem Soc 77(14):3701–3707
Wungtanagorn R, Schmidt SJ (2001a) Thermodynamic properties and kinetics of the physical aging of amorphous glucose, fructose and their mixture. J Therm Anal Calorim 65:9–35
Wungtanagorn R, Schmidt SJ (2001b) Phenomenological study of enthalpy relaxation of amorphous glucose, fructose, and their mixture. Thermochim Acta 369:95–116
Yoshida H (1995) Relationship between enthalpy relaxation and dynamic mechanical relaxation of engineering plastics. Thermochim Acta 266:119–127
Yu L (2001) Amorphous pharmaceutical solids: Preparation, characterization and stabilization. Adv Drug Deliv Rev 48(1):27–42
Acknowledgements
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, F., Roos, Y.H. Glass Transition-Associated Structural Relaxations and Applications of Relaxation Times in Amorphous Food Solids: a Review. Food Eng Rev 9, 257–270 (2017). https://doi.org/10.1007/s12393-017-9166-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-017-9166-6