Abstract
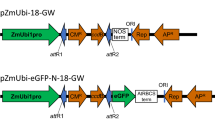
Transient gene expression systems using protoplasts have been widely used for rapid functional characterization of genes in many plant species. Brachypodium distachyon has recently been employed as a model plant for studies on biofuel grass species and grass crops because of its small genome size, short life-span, and availability of efficient transformation systems. Here, we report the an efficient protocol for the preparation of leaf mesophyll protoplasts from Brachypodium seedlings. We also modified the polyethylene glycol (PEG)-mediated transformation procedure to optimize experimental conditions, such as duration of enzyme digestion, PEG incubation time, and plasmid DNA concentration and size. The green fluorescence protein (GFP)- and β-glucuronidase (GUS)-coding genes were used as reporters to evaluate the feasibility of this transient expression system. We found that the yield of viable protoplasts was highest after 3 h of enzymatic digestion. Viability of enzyme-digested protoplasts was moderately maintained up to 24 h in Mmg preincubation solution. In addition, the highest transient expression of reporter genes was obtained when protoplasts were transformed with 20 μg of plasmid DNA and incubated for 16 h. Together with the recent completion of the Brachypodium genome sequence, the Brachypodium transient expression system using leaf mesophyll protoplasts can be widely used for cellular, molecular, and biochemical studies of genes involved in carbon metabolism and signaling pathways mediating intrinsic and environmental cues.
Similar content being viewed by others
References
Abel S, Theologis A (1994) Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. Plant J 5:421–427
Abel S, Theologis A (1996) Early genes and auxin action. Plant Physiol 111:9–17
Bart R, Chern M, Park CJ, Bartley L, Ronald PC (2006) A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts. Plant Methods 2:13–22
Binder KA, Wegner LH, Heidecker M, Zimmermann U (2003) Gating of Cl− currents in protoplasts from the marine alga Valonia utricularis depends on the transmembrane Cl− gradient and is affected by enzymatic cell wall degradation. J Membr Biol 191:165–178
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brkljacic J, Grotewold E, Scholl R, Mockler T, Garvin DF, Vain P, Brutnell T, Sibout R, Bevan M, Budak H, Caicedo AL, Gao C, Gu Y, Hazen SP, Holt BF 3rd, Hong SY, Jordan M, Manzaneda AJ, Mitchell-Olds T, Mochida K, Mur LA, Park CM, Sedbrook J, Watt M, Zheng SJ, Vogel JP (2011) Brachypodium as a model for the grasses: today and the future. Plant Physiol 157:3–13
Bruce WB, Christensen AH, Klein T, Fromm M, Quail PH (1989) Photoregulation of a phytochrome gene promoter from oat transferred into rice by particle bombardment. Proc Natl Acad Sci USA 86:9692–9696
Chang CC, Sheen J, Bligny M, Niwa Y, Lerbs-Mache S, Stern DB (1999) Functional analysis of two maize cDNAs encoding T7-like RNA polymerases. Plant Cell 11:911–926
Chen S, Tao L, Zeng L, Vega-Sanchez ME, Umemura K, Wang GL (2006) A highly efficient transient protoplast system for analyzing defence gene expression and protein-protein interactions in rice. Mol Plant Pathol 7:417–427
Datla RS, Hammerlindl JK, Panchuk B, Pelcher LE, Keller W (1992) Modified binary plant transformation vectors with the wild-type gene encoding NPTII. Gene 122:383–384
Datta K, Datta SK (1999) Transformation of rice via PEG-mediated DNA uptake into protoplasts. Methods Mol Biol 111:335–347
Davey MR, Anthony P, Power JB, Lowe KC (2004) Protoplast applications in biotechnology. In: Goodman RM (ed) Encyclopaedia of plant and crop science. Marcel Dekker Inc., New York, pp 1061–1064
Davey MR, Anthony P, Power JB, Lowe KC (2005) Plant protoplasts: status and biotechnological perspectives. Biotechnol Adv 23:131–171
Davey MR, Anthony P, Power JB, Lowe KC (2006) Isolation, culture, and plant regeneration from leaf protoplasts of Passiflora. Methods Mol Biol 318:201–210
Davey MR, Kumar A (1983) Higher plant protoplasts-retrospect and prospect. Int Rev Cytol Suppl 16:219–299
De Domenico S, Bonsegna S, Lenucci MS, Poltronieri P, Di Sansebastiano GP, Santino A (2011) Localization of seed oil body proteins in tobacco protoplasts reveals specific mechanisms of protein targeting to leaf lipid droplets. J Integr Plant Biol 53:858–868
De Sutter V, Vanderhaeghen R, Tilleman S, Lammertyn F, Vanhoutte I, Karimi M, Inzé D, Goossens A, Hilson P (2005) Exploration of jasmonate signalling via automated and standardized transient expression assays in tobacco cells. Plant J 44:1065–1076
Draper J, Mur LA, Jenkins G, Ghosh-Biswas GC, Bablak P, Hasterok R, Routledge AP (2001) Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiol 127:1539–1555
Elliott AR, Campbell JA, Dugdale B, Brettell, RS, Grof CL (1999) Green fluorescent protein facilitates rapid in vivo detection of genetically transformed plant cells. Plant Cell Rep 18:707–714
Evans DA, Bravo JE (1983) Protoplast isolation and culture. In: Evans DA, Sharp WR, Ammirato PV, Yamada Y (eds) Handbook of Plant Cell Culture. MacMillan, New York, pp 124–176
Filiz E, Ozdemir BS, Budak F, Vogel JP, Tuna M, Budak H (2009) Molecular, morphological, and cytological analysis of diverse Brachypodium distachyon inbred lines. Genome 52:876–890
Fischer R, Hain R (1995) Tobacco protoplast transformation and use for functional analysis of newly isolated genes and gene constructs. Methods Cell Biol 50:401–410
Frohnmeyer H, Hahlbrock K, Schafer E (1994) A light responsive in vitro transcription system from evacuolated parsley protoplasts. Plant J 5:437–449
Goodin MM, Dietzgen RG, Schichnes D, Ruzin S, Jackson AO (2002) pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J 31:375–383
Hansen G, Wright MS (1999) Recent advances in the transformation of plants. Trends Plant Sci 4:226–231
Hayashimoto A, Li Z, Murai N (1990) A polyethylene glycol-mediated protoplast transformation system for production of fertile transgenic rice plants. Plant Physiol 93:857–863
Hoffman A, Halfter U, Morris PC (1994) Transient expression in leaf mesophyll protoplasts of Arabidopsis thaliana. Plant Cell Tiss Org Cult 36:53–58
Hong SY, Park JH, Cho SH, Yang MS, Park CM (2011) Phenological growth stages of Brachypodium distachyon: codification and description according to the BBCH scale. Weed Res 51:612–620
Hong SY, Seo PJ, Yang MS, Xiang F, Park CM (2008) Exploring valid reference genes for gene expression studies in Brachypodium distachyon by real-time PCR. BMC Plant Biol 8:112–122
Hrazdina G, Marx GA, Hoch HC (1982) Distribution of secondary plant metabolites and their biosynthetic enzymes in pea (Pisum sativum L.) leaves: anthocyanins and flavonol glycosides. Plant Physiol 70:745–748
Huang CN, Cornejo MJ, Bush DS, Jones RL (1986) Estimating viability of plant protoplasts using double and single staining. Protoplasma 135:80–87
Huo N, Gu Y, Lazo G, Vogel J, Coleman-Derr D, Luo M, Thilmony R, Garvin D, Anderson O (2006) Construction and characterization of two BAC libraries from Brachypodium distachyon, a new model for grass genomics. Genome 49:1099–1108
International Brachypodium Initiative (2010) Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 463:763–768
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jin JB, Kim YA, Kim SJ, Lee SH, Kim DH, Cheong GW, Hwang I (2001) A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13:1511–1526
Kim MJ, Baek K, Park CM (2009) Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep 28:1159–1167
Koop HU, Steinmüller K, Wagner H, Rössler C, Eibl C, Sacher L (1996) Integration of foreign sequences into the tobacco plastome via polyethylene glycol-mediated protoplast transformation. Planta 199:193–201
Larkin PJ (1976) Purification and viability of plant protoplasts. Planta 128:213–216
Li JF, Nebenführ A (2010) FAST technique for Agrobacterium-mediated transient gene expression in seedlings of Arabidopsis and other plant species. Cold Spring Harb Protoc 5:5428–5431
Liu CN, Li XQ, Gelvin SB (1992) Multiple copies of virG enhance the transient transformation of celery, carrot and rice tissues by Agrobacterium tumefaciens. Plant Mol Biol 20:1071–1087
Loake GJ, Choudhary AD, Harrison MJ, Mavandad M, Lamb CJ, Dixon RA (1991) Phenylpropanoid pathway intermediates regulate transient expression of a chalcone synthase gene promoter. Plant Cell 3:829–840
Locatelli F, Vannini C, Magnani E, Coraggio I, Bracale M (2003) Efficiency of transient transformation in tobacco protoplasts is independent of plasmid amount. Plant Cell Rep 21:865–871
Luehrsen KR, de Wet JR, Walbot V (1992) Transient expression analysis in plants using firefly luciferase reporter gene. Methods Enzymol 216:397–414
Maas C, Werr W (1989) Mechanism and optimized conditions for PEG mediated DNA transfection into plant protoplasts. Plant Cell Rep 8:148–151
Manavella PA, Chan RL (2009) Transient transformation of sunflower leaf discs via an Agrobacterium-mediated method: applications for gene expression and silencing studies. Nat Protoc 4:1699–1707
Massa AN, Wanjugi H, Deal KR, O’Brien K, You FM, Maiti R, Chan AP, Gu YQ, Luo MC, Anderson OD, Rabinowicz PD, Dvorak J, Devos KM (2011) Gene space dynamics during the evolution of Aegilops tauschii, Brachypodium distachyon, Oryza sativa, and Sorghum bicolor genomes. Mol Biol Evol 28:2537–2547
Mathur J, Koncz C (1998) PEG-mediated protoplast transformation with naked DNA. Methods Mol Biol 82:267–276
Mazarei M, Al-Ahmad H, Rudis MR, Stewart CN Jr (2008) Protoplast isolation and transient gene expression in switchgrass, Panicum virgatum L. Biotechnol J 3:354–359
Mitsuhara I, Ugaki M, Hirochika H, Ohshima M, Murakami T, Gotoh Y, Katayose Y, Nakamura S, Honkura R, Nishimiya S, Ueno K, Mochizuki A, Tanimoto H, Tsugawa H, Otsuki Y, Ohashi Y (1996) Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol 37:49–59
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue culture. Physiol Plant 15:473–497
Nimchuk Z, Marois E, Kjemtrup S, Leister RT, Katagiri F, Dangl JL (2000) Eukaryotic fatty acylation drives plasma membrane targeting and enhances function of several type III effector proteins from Pseudomonas syringae. Cell 101:353–363
Nurnberger T, Nennstiel D, Jabs T, Sacks WR, Hahlbrock K, Scheel D (1994) High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell 78:449–460
Opanowicz M, Vain P, Draper J, Parker D, Doonan JH (2008) Brachypodium distachyon: making hay with a wild grass. Trends Plant Sci 13:172–177
Power JB, Cocking EC (1970) Isolation of leaf protoplasts: macromolecular uptake and growth substance response. J Exp Bot 21:64–70
Rao AQ, Bakhsh A, Kiani S, Shahzad K, Shahid AA, Husnain T, Riazuddin S (2009) The myth of plant transformation. Biotechnol Adv 27:753–763
Rasmussen JO, Rasmussen OS (1993) PEG mediated DNA uptake and transient GUS expression in carrot, rapeseed and soybean protoplasts. Plant Sci 89:199–207
Sheen J (1999) C4 gene expression. Ann Rev Plant Physiol Plant Mol Biol 50:187–217
Sheen J (2001) Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol 127:1466–1475
Subramaniam R, Desveaux D, Spickler C, Michnick SW, Brisson N (2001) Direct visualization of protein interactions in plant cells. Nat Biotechnol 19:769–772
Swanson SJ, Bethke PC, Jones RL (1998) Barley aleurone cells contain two types of vacuoles. Characterization of lytic organelles by use of fluorescent probes. Plant Cell 10:685–698
Ueki S, Lacroix B, Krichevsky A, Lazarowitz SG, Citovsky V (2009) Functional transient genetic transformation of Arabidopsis leaves by biolistic bombardment. Nat Protoc 4:71–77
Ulmasov T, Hagen G, Guilfoyle TJ (1999) Activation and repression of transcription by auxin-response factors. Proc Natl Acad Sci USA 96:5844–5849
Unver T, Budak H (2009) Conserved microRNAs and their targets in model grass species Brachypodium distachyon. Planta 230:659–669
Vain P, Worland B, Thole V, McKenzie N, Alves SC, Opanowicz M, Fish LJ, Bevan MW, Snape JW (2008) Agrobacterium-mediated transformation of the temperate grass Brachypodium distachyon (genotype Bd21) for T-DNA insertional mutagenesis. Plant Biotechnol J 6:236–245
Vogel J, Bragg J (2009) Brachypodium distachyon, a new model for the Triticeae. In: Feuillet C, Muehlbauer GJ (eds) Genetics and Genomics of the Triticeae. Springer, New York, pp 427–449
Vogel J, Hill T (2008) High-efficiency Agrobacterium-mediated transformation of Brachypodium distachyon inbred line Bd21-3. Plant Cell Rep 27:471–478
Vogel JP, Garvin DF, Leong OM, Hayden DM (2006) Agrobacterium-mediated transformation and inbred line development in the model grass Brachypodium distachyon. Plant Cell Tiss Org Cult 85:199–211
Wagner GJ, Butcher IV WC, Siegelman NW (1978) The plant protoplast: A useful tool for plant research and student instruction. Bioscience 28:95–101
Widholm JM (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:189–194
Worley CK, Zenser N, Ramos J, Rouse D, Leyser O, Theologis A, Callis J (2000) Degradation of Aux/IAA proteins is essential for normal auxin signalling. Plant J 21:553–562
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565–1572
Zhang Y, Su J, Duan S, Ao Y, Dai J, Liu J, Wang P, Li Y, Liu B, Feng D, Wang J, Wang H (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7:30–44
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, SY., Seo, P.J., Cho, SH. et al. Preparation of leaf mesophyll protoplasts for transient gene expression in Brachypodium distachyon . J. Plant Biol. 55, 390–397 (2012). https://doi.org/10.1007/s12374-012-0159-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-012-0159-y