Abstract
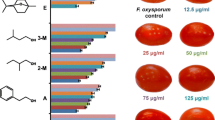
We investigated variations in the level and composition of volatiles emitted by tomato leaves at different ages. Our focus also included their antifungal properties and responses to chitosan oligosaccharide. Based on leaf position, the release of volatiles decreased over time. Young leaves produced high levels of C6-aldehyde, which is mainly composed of hexenal, while the volatiles emitted by more mature leaves largely comprised terpenes, particularly β-phellandrene and caryophyllane. In young upper leaves, the main components (up to 86% of the total) were hexenal, β-phellandrene, and caryophyllane. Their levels decreased steadily over time, from 386.3 μg g−1 fresh weight (FW) in young leaves to 113.2 μg g−1 FW in old tissues. Volatiles emitted from young leaves exhibited the best antifungal activity against spore germination and hyphal growth by Botrytis cinerea and Fusarium oxysporum. Leaves became more susceptible to oligosaccharide treatment with increasing age. When young tissues were exposed to chitosan, we found declines in both the quantity of volatiles and their ability to inhibit fungal growth. Compared with the control, the amount of volatiles from young tissues was 88.4% lower after such treatment. In contrast, contents of volatiles from old and adult leaves were dramatically increased by chitosan oligosaccharide. Likewise, their inhibitory effect was significantly enhanced. Therefore, our results suggest that these volatiles are responsible for antifungal activity and may play a role in age-related resistance by tomato.



Similar content being viewed by others
References
Cardoza YJ, Tumlinson JH (2006) Compatible and incompatible Xanthomonas infection differentially affect herbivore-induced volatile emission by pepper plants. J Chem Ecol 32:1755–1768
Cardoza YJ, Alborn HT, Tumlinson JH (2002) In vivo volatile emissions from peanut plants induced by simultaneous fungal infection and insect damage. J Chem Ecol 28:161–174
Dudai N, Larkov O, Ravid U, Putievsky E, Lewinsohn E (2001) Developmental control of monoterpene content and composition in Micromeria fruticosa (L.) Druce. Ann Bot 88:349–354
Dudareva N, Negre F (2005) Practical applications of research into the regulation of plant volatile emission. Curr Opin Plant Biol 8:113–118
Dudareva N, Pichersky E, Gershenzon J (2004) Biochemistry of plant volatiles. Plant Physiol 135:1893–1902
Farmer EE (2001) Surface-to-air signals. Nature 411:854–856
Hamilton-Kemp TR, McCracken CT, Loughrin JH, Andersen RA, Hildebrand DF (1992) Effects of some natural volatile compounds on the pathogenic fungi Alternaria alternata and Botrytis cinerea. J Chem Ecol 18(7):1083–1086
He PQ, Zhang PY, Chen KS, Li GY (2005a) Inhibitory effects of several volatiles of Lycopersicon esculentum on Botrytis cinerea. Acta Bot Yunnanica 27:315–320 (in Chinese with an English abstract)
He PQ, Lin XZ, Shen JH, Huang XH, Chen KS, Li GY (2005b) Induction of volatile organic compounds in the leaves of Lycopersicon esculentum by chitosan oligomer. High Technol Lett 11:95–100
Holopainen JK (2004) Multiple functions of inducible plant volatiles. Trends Plant Sci 9(11):529–533
Hu ZH, Shen YB, Luo YQ, Shen FY, Gao HB, Gao RF (2008) Aldehyde volatiles emitted in succession from mechanically damaged leaves of poplar cuttings. J Plant Biol 51(4):269–275
Huang J, Cardoza YJ, Schmelz EA, Raina R, Englberth J, Tumlinson JH (2003) Differential volatile emissions and salicylic acid levels from tobacco plants in response to different strains of Pseudomonas syringae. Planta 217:767–775
Kus JV, Zaton K, Sarkar R, Carmeron RK (2002) Age-related resistance in Arabidopsis is a developmentally regulated defense response to Pseudomonas syringae. Plant Cell 14:479–490
Kuzma J, Fall R (1993) Leaf isoprene emission rate is dependent on leaf development and the level of isoprene synthase. Plant Physiol 101:435–440
Kwon SI, Cho HJ, Bae K, Jung HJ, Jin HC, Park OK (2009) Role of an Arabidopsis Rab GTPase RabG3b in pathogen response and leaf senescence. J Plant Biol 52:79–87
Lauge R, Dmitriev AP, Joosten MHAJ, de Wit PJGM (1998) Additional resistance genes against Cladosporium fulvum present on the Cf9 introgression segment are associated with strong PR protein accumulation. Mol Plant Microb Interact 11:301–308
Moalemiyan M, Vikram A, Kushalappa AC (2007) Detection and discrimination of two fungal diseases of mango (cv. Keitt) fruits based on volatile metabolite profiles using GC/MS. Postharvest Biol Technol 45:117–125
Obara N, Hasegawa M, Kodama O (2002) Induced volatiles in elicitor-treated and rice blast fungus-inoculated rice leaves. Biosci Biotechnol Biochem 66:2549–2559
Panter SN, Jones DA (2002) Age-related resistance to plant pathogens. Adv Bot Res 38:252–280
Panter SN, Hammond-Kosack KE, Harrison K, Jones JDG, Jones DA (2002) Developmental control of promoter activity is not responsible for mature onset of Cf-9B-mediated resistance to leaf mold in tomato. Mol Plant Microb Interact 15:1099–1107
Park JM, Paek KH (2007) Recognition and response in plant–pathogen interactions. J Plant Biol 50(2):132–138
Parniske M, Hammond-Kosack KE, Golstein C, Thomas CM, Jones DA, Harrison K, Wulff BBH, Jones JDG (1997) Novel disease resistance specificities result from sequence exchange between tandemly repeated genes at the Cf-4/9 locus of tomato. Cell 91:821–832
Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5(3):237–243
Rusterucci C, Zhao Z, Haines K, Mellersh D, Neumann M, Cameron RK (2005) Age-related resistance to Pseudomonas syringae pv. tomato is associated with the transition to flowering in Arabidopsis and is effective against Peronospora parasitica. Physiol Mol Plant Pathol 66:222–231
Shulaev V, Silverman P, Raskin I (1997) Airborne signaling by methyl salicylate in plant pathogen resistance. Nature 385:718–721
Staudt M, Bertin N, Hansen U, Seufert G, Ciccioli P, Foster P, Frenzel B, Fugit JL (1997) Seasonal and diurnal patterns of monoterpene emission from Pinus pinea (L.) under field conditions. Atmos Environ 31:145–156
Yun KW, Choi SK (2003) Seasonal variation in allelopathic potential of Artemisia princeps var. orientalis on plants and microbes. J Plant Biol 46(2):105–110
Zeier J (2005) Age-dependent variations of local and systemic defence responses in Arabidopsis leaves towards an avirulent strain of Pseudomonas syringae. Physiol Mol Plant Pathol 66:30–39
Zhang PY, He PQ, Chen KS, Xie HB (2006) Inhibitory effects of several volatile organic compounds of Lycopersicon esculentum on Fusarium oxysporum f. sp flycoporsici Sacc. Acta Phytopathol Sin 1:91–93 (in Chinese with an English Name)
Zhang PY, Chen KS, He PQ, Liu SH, Jiang WF (2008) Effects of crop development on the emission of volatile in tomato leaves and its inhibitory activity to Botrytis cinerea Pers. and Fusarium oxysporum Schl. J Integrat Plant Biol 50(1):84–91
Zhuang H, Hamilton-Kemp TR, Andersen RA, Hildebrand DF (1992) Developmental change in C6-aldehyde formation by soybean leaves. Plant Physiol 100:80–87
Acknowledgments
This research was financially supported by the Hi-Tech Research and Development (863) Program of China (No. 2007AA10Z334) and the Project of Science and Technology Development Plan in Shandong Province (2008GG10009014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, P., Chen, K. Age-Dependent Variations of Volatile Emissions and Inhibitory Activity Toward Botrytis cinerea and Fusarium oxysporum in Tomato Leaves Treated with Chitosan Oligosaccharide. J. Plant Biol. 52, 332–339 (2009). https://doi.org/10.1007/s12374-009-9043-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-009-9043-9