Abstract
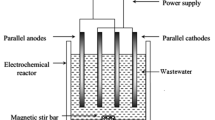
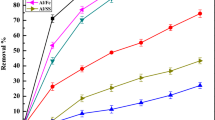
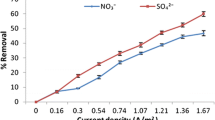
Sugar industries generate highly polluted effluent in terms of organics and inorganics load and it is very difficult to treat. In the present study, attempts have been made to use electrocoagulation process to treat the effluent. The treatment was carried out using different electrode materials in different combinations. The influence of pH, current density (CD) and treatment time (tR) on COD removal of sugar industry effluent (SIE) was evaluated. Apart from this, electrode loss, species energy consumption and cost analysis were also carried out. For all electrode materials, pH 7 was found to be optimum, and CD in range of 69.44–104.165 A/m2 provided good treatment of the effluent. The performance of Al–MS electrode combination was seen to be the best, as it reduced initial COD of SIE = 4800 mg/dm3 to 920 mg/dm3 (80.83% COD reduction) in 100 min, with treatment cost INR 326 (USD 4.90)/m3 SIE. Kinetics studies showed COD reduction rate to 1.5 order with respect to COD and 0.57 order with respect to CD. The reaction rate constant lies in between 5.0 and 7.1 \( \left( {\frac{{{\text{dm}}^{3} }}{\text{mg}}} \right)^{0.5 } \left( {\frac{{{\text{m}}^{2} }}{\text{A}}} \right)^{0.57} \,\min^{ - 1} \).





Similar content being viewed by others
References
Adhoum, N., and L. Monser. 2004. Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chemical Engineering and Processing 43: 1281–1287.
Can, O.T., M. Bayramoglu, and M. Kobya. 2003. Decolorization of reactive dye solutions by electrocoagulation using aluminum electrodes. Industrial and Engineering Chemistry Research 42: 3391–3396.
Can, O.T., M. Kobya, E. Demirbas, and M. Bayramoglu. 2006. Treatment of the textile wastewater by combined electrocoagulation. Chemosphere 62: 181–187.
Chaudhari, P.K., I.M. Mishra, and S. Chand. 2007. Decolourization and removal of chemical oxygen demand (COD) with energy recovery: Treatment of biodigester effluent of a molasses-based alcohol distillery using inorganic coagulants. Colloids and Surfaces A: Physicochemical and Engineering Aspects 296: 238–247.
Guven, G., A. Perendeci, and A. Tanyolac. 2009. Electrochemical treatment of simulated beet sugar factory wastewater. Chemical Engineering Journal 151: 149–159.
Katal, R., and H. Pahlavanzadeh. 2011. Influence of different combinations of aluminum and iron electrode on electrocoagulation efficiency: Application to the treatment of paper mill wastewater. Desalination 265: 199–205.
Kobya, M., O.T. Can, and M. Bayramoglu. 2003. Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. Journal of Hazardous Materials 100: 163–178.
Kobya, M., and S. Delipinar. 2008. Treatment of the baker’s yeast wastewater by electrocoagulation. Journal of Hazardous Materials 154: 1133–1140.
Kobya, M., and E. Demirbas. 2015. Evaluations of operating parameters on treatment of can manufacturing wastewater by electrocoagulation. Journal of Water Process Engineering 8: 64–74.
Kumar, A., P.V. Nidheesh, and M.S. Kumar. 2018. Composite wastewater treatment by aerated electrocoagulation and modified peroxi-coagulation processes. Chemosphere 205: 587–593.
Kushwaha, J.P. 2013. A review on sugar industry wastewater: Sources, treatment technologies, and reuse. Desalination and Water Treatment 53: 309–318.
Mahesh, S., B. Prasad, I.D. Mall, and I.M. Mishra. 2006. Electrochemical degradation of pulp and paper mill wastewater. Part 2. Characterization and analysis of sludge. Industrial and Engineering Chemistry Research 45: 5766–5774.
Migo, V.P., M. Matsumura, E.J.D. Rosario, and H. Kataoka. 1993. Decolorization of molasses wastewater using an inorganic flocculant. Journal of Bioscience and Bioengineering 75 (6): 438–442.
Moraes, B.S., M. Zaiat, and A. Bonomi. 2015. Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: Challenges and perspectives. Renewable and Sustainable Energy Reviews 44: 888–903.
Nawarkar, C.J., and V.D. Salkar. 2019. Solar powered electrocoagulation system for municipal wastewater treatment. Fuel 237: 222–226.
Prajapati, A.K., and P.K. Chaudhari. 2014. Electrochemical treatment of rice grain-based distillery effluent: Chemical oxygen demand and colour removal. Environmental Technology 35: 242–249.
Prajapati, A.K., and P.K. Chaudhari. 2015. Physicochemical treatment of distillery wastewater—A review. Chemical Engineering Communications 202: 1098–1117.
Rice, E.W., R.B. Baired, A.D. Eaton, and L.S. Clesceri. 2012. Standard methods for the examination of water and wastewater, 22nd ed. Troy: Washington DC.
Sahu, O. 2018. Assessment of sugarcane industry: Suitability for production, consumption, and utilization. Annals of Agrarian Science 16: 389–395.
Sahu, O.P., and P.K. Chaudhari. 2015. Electrochemical treatment of sugar industry wastewater: COD and color removal. Journal of Electroanalytical Chemistry 739: 122–129.
Sahu, O.P., V. Gupta, P.K. Chaudhari, and V.C. Srivastava. 2015. Electrochemical treatment of actual sugar industry wastewater using aluminum electrode. International Journal of Environmental Science and Technology 12: 3519–3530.
Shivayogimath, C.B., and R. Jahagirdar. 2013. Treatment of sugar industry wastewater using electrocoagulation technique. International Journal of Engineering Research and Technology 1: 262–265.
Tiwari, A., and O.P. Sahu. 2017. Treatment of food-agro (sugar) industry wastewater with copper metal and salt: Chemical oxidation and electro-oxidation combined study in batch mode. Water Resources and Industry 17: 19–25.
www.indiabrifing.com. Accessed 10 December 2018.
www.indiamart.com. Accessed 10 December 2018.
www.isosugar.org. Accessed 20 January 2019.
Yavuz, Y., and Ü.B. Ögütveren. 2018. Treatment of industrial estate wastewater by the application of electrocoagulation process using iron electrodes. Journal of Environmental Management 207: 151–158.
Acknowledgements
Authors are grateful to SERB, DST, New Delhi, for providing fund to research under research grant: File No: EEQ/2016/000068.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gondudey, S., Chaudhari, P.K. Influence of Various Electrode Materials in Electrocoagulation Efficiency: Application in Treatment of Sugar Industry Effluent. Sugar Tech 22, 15–27 (2020). https://doi.org/10.1007/s12355-019-00753-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-019-00753-6