Abstract
Background
82Rb kinetics may distinguish scar from viable but dysfunctional (hibernating) myocardium. We sought to define the relationship between 82Rb kinetics and myocardial viability compared with conventional 82Rb and 18F-fluorodeoxyglucose (FDG) perfusion-metabolism PET imaging.
Methods
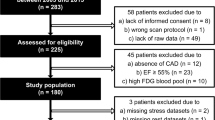
Consecutive patients (N = 120) referred for evaluation of myocardial viability prior to revascularization and normal volunteers (N = 37) were reviewed. Dynamic 82Rb 3D PET data were acquired at rest. 18F-FDG 3D PET data were acquired after metabolic preparation using a standardized hyperinsulinemic-euglycemic clamp. 82Rb kinetic parameters K1, k2, and partition coefficient (KP) were estimated by compartmental modeling
Results
Segmental 82Rb k2 and KP differed significantly between scarred and hibernating segments identified by Rb-FDG perfusion-metabolism (k2, 0.42 ± 0.25 vs. 0.22 ± 0.09 min−1; P < .0001; KP, 1.33 ± 0.62 vs. 2.25 ± 0.98 ml/g; P < .0001). As compared to Rb-FDG analysis, segmental Rb KP had a c-index, sensitivity and specificity of 0.809, 76% and 84%, respectively, for distinguishing hibernating and scarred segments. Segmental k2 performed similarly, but with lower specificity (75%, P < .001)
Conclusions
In this pilot study, 82Rb kinetic parameters k2 and KP, which are readily estimated using a compartmental model commonly used for myocardial blood flow, reliably differentiated hibernating myocardium and scar. Further study is necessary to evaluate their clinical utility for predicting benefit after revascularization.
Spanish Abstract
Antecedentes
La cinética con 82Rb puede distinguir las cicatrices miocárdicas del tejido viable pero disfuncional (hibernante). Buscamos definir la relación entre la cinética del 82Rb y la viabilidad miocárdica comparada con las imágenes convencionales de PET con 82Rb y 18F-flurodeoxyglucosa (FDG).
Métodos
se evaluaron a Pacientes Consecutivos (N = 120) referidos para evaluación de viabilidad miocárdica previos a revascularización y voluntarios normales (N = 37). Los datos de PET 3D dinámico con 82Rb fueron adquiridos en reposo. Los datos de 18F-FDG 3D PET fueron adquiridos posterior a la preparación metabólica usando un clamp hiperinsulínico-euglucémico. Los patrones cinéticos K1, k2 y el coeficiente de partición (KP) fueron estimados por el modelo compartimental.
Resultados
el k2 y KP del 82Rb permitueron distinguier el tejido cicatricial de los segmentos hibernados identificados por la vía perfusión metabolismo de Rb-FDG (k2, 0.42 ± 0.25 vs. 0.22 ± 0.09 min-1; P < .0001; KP, 1.33 ± 0.62 vs. 2.25 ± 0.98 ml/g; P < .0001). En comparación con el análisis de Rb-FDG, el KP segmentario del Rb tuvo un índice-c, sensbilidad y especificidad de 0.809 76 y 84%, respectivamente, para distinguir segmentos hibernantes y cicatriciales. k2 Segmentario tuvo un desempeño similar pero con una menor especificidad (75%, P < .001).
Conclusiones
En este estudio piloto, los parámetros cinéticos k2 y KP del 82Rb, los cuales son estimados usando un modelo compartimental usado comúnmente para flujo sanguíneo miocárdico, diferenciaron el miocardio hibernante del cicatricial. Se necesita estudiar más a fondo para evaluar la utilidad clínica para la predicción del beneficio posterior a la revascularización
Chinese Abstract
背景
铷-82(82Rb)药代动力学可能区分梗死与功能受损但存活的心肌(冬眠心肌)。我们将探讨82Rb药代动力学与存活心肌的关系,并与常规的82Rb和18F-氟脱氧葡萄糖(FDG)灌注-代谢PET显像进行比较。
方法
连续纳入120名血运重建前评估存活心肌的患者和37名正常志愿者。获得静息82Rb 3D-PET动态采集数据;采用标准化的高胰岛素 - 正葡萄糖钳夹试验得到18F-FDG 3D PET代谢显像数据; 通过房室模型计算82Rb动力学参数k1,k2和分配系数(KP)。
结果
以Rb-FDG灌注代谢显像鉴定疤痕心肌组和冬眠心肌组,两组的82Rb节段性 k2值和KP值有明显差异(k2, 0.42 ± 0.25 vs 0.22 ± 0.09 min−1; P < .0001; KP, 1.33 ± 0.62 vs 2.25 ± 0.98 ml/g; P < .0001)。以Rb-FDG灌注代谢显像为诊断标准,82Rb节段性 KP对疤痕和冬眠心肌鉴别效能的c指数,敏感性和特异性分别是0.809, 76%和84%。节段性k2的鉴别效能与KP相似,但特异性较低(75%,P < .001)。
结论
在本试验性研究中,通过常规用于82Rb心肌血流量测定的房室模型计算出的82Rb动力学参数k2和KP,可以有效的区分冬眠心肌和瘢痕心肌。 但需要进一步的研究来评估其对预测血运重建是否获益的临床价值。
French Abstract
Contexte
La cinétique du 82Rb pourrait être utilisée pour distinguer la fibrose myocardique des zones viables mais dysfonctionnelles (en hibernation). Dans cette étude, nous avons cherché à définir la relation entre la cinétique du 82Rb et la viabilité myocardique en comparant les images de perfusion et métabolisme myocardique utilisant le 82Rb et le 18F-fluorodésoxyglucose (FDG)
Méthodes
les données obtenues chez 120 patients consécutifs (N = 120) référés pour évaluation de la viabilité du myocarde avant revascularisation et 37 volontaires considérés normaux (N = 37) ont été étudieés. Les données TEP 3D dynamiques 82Rb ont été acquises au repos. Les données TEP 3D au 18F-FDG ont été acquises après préparation métabolique utilisant une pince hyperinsulémique-euglycémique normalisée. Les paramètres k1, k2 et le coefficient de partage (KP) de la cinétique du 82R bont été évalues par modélisation compartimentale.
Résultats
les paramètres k2 et KP de distribution du 82Rb se sont révélés significativement différent entre les zones tissulaires cicatricielles fibreuses et celles en hibernation identifiées par étude métabolique au FDG (k2, 0,42 ± 0,25 vs. 0,22 ± 0,09 min−1; P < 0,0001; KP, 1,33 ± 0,62 vs. 2,25 ± 0,98 ml/g; P < 0,0001). Par rapport aux données FDG, le paramètre KP du rubidium a révélé un indice de concordance de 0,809 et une sensibilité et spécificité de 76% et 84%, pour la distinction des segments en hibernation et cicatrisés. Les résultats du paramètre k2 se sont révélés similaires, mais avec une spécificité plus faible calculée à 75% (P < 0,001).
Conclusions
Dans cette étude pilote, les paramètres cinétiques k2 et KP de perfusion myocardique au 82Rb, qui sont facilement estimés à l’aide d’un modèle compartimental couramment utilisé pour flux sanguin myocardique, ont permis de différencier de manière fiable le myocarde cicatricielle des zones myocardiques hibernantes. Une étude plus approfondie est souhaitable pour valider leur utilité clinique en revacularisation.




Similar content being viewed by others
Abbreviations
- PET:
-
Positron emission tomography
- 82Rb:
-
Rubidium-82
- 18F:
-
Fluorine-18
- FDG:
-
Fluorodeoxyglucose
- ICM:
-
Ischemic cardiomyopathy
- GIR:
-
Glucose infusion rate
References
Anavekar NS, Chareonthaitawee P, Narula J, Gersh BJ. Revascularization in patients with severe left ventricular dysfunction: is the assessment of viability still viable? J Am Coll Cardiol 2016;67(24):2874-87.
Schinkel AFL, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol 2007;32(7):375-410.
Allman KC. Noninvasive assessment myocardial viability: Current status and future directions. J Nucl Cardiol 2013;20(4):618-37.
Schelbert HR. Positron emission tomography of the heart: Methodology, findings in the normal and the diseased heart, and clinical applications. In: Phelps ME, editor. PET: Molecular Imaging and Its Biological Applications. 1st ed. New York: Springer-Verlag; 2004. p. 389-508.
Goldstein RA. Kinetics of rubidium-82 after coronary occlusion and reperfusion. Assessment of patency and viability in open-chested dogs. J Clin Invest 1985;75(4):1131-1137.
Goldstein RA. Rubidium-82 kinetics after coronary occlusion: temporal relation of net myocardial accumulation and viability in open-chested dogs. J Nucl Med 1986;27(9):1456-61.
Gould KL, Yoshida K, Hess MJ, Haynie M, Mullani N, Smalling RW. Myocardial metabolism of fluorodeoxyglucose compared to cell membrane integrity for the potassium analogue rubidium-82 for assessing infarct size in man by PET. J Nucl Med 1991;32(1):1-9.
Yoshida K, Gould KL. Quantitative relation of myocardial infarct size and myocardial viability by positron emission tomography to left ventricular ejection fraction and 3-year mortality with and without revascularization. J Am Coll Cardiol 1993;22(4):984-97.
vom Dahl J, Muzik O, Wolfe ER, Allman C, Hutchins G, Schwaiger M. Myocardial rubidium-82 tissue kinetics assessed by dynamic positron emission tomography as a marker of myocardial cell membrane integrity and viability. Circulation 1996;93(2):238-45.
Stankewicz MA, Mansour CS, Eisner RL, et al. Myocardial viability assessment by PET: 82Rb defect washout does not predict the results of metabolic-perfusion mismatch. J Nucl Med 2005;46(10):1602-9.
Chien DT, Bravo P, Higuchi T, Merrill J, Bengel FM. Washout of 82Rb as a marker of impaired tissue integrity, obtained by list-mode cardiac PET/CT: relationship with perfusion/metabolism patterns of myocardial viability. Eur J Nucl Med Mol Imaging 2011;38(8):1507-15.
Moody JB, Lee BC, Corbett JR, Ficaro EP, Murthy VL. Precision and accuracy of clinical quantification of myocardial blood flow by dynamic PET: A technical perspective. J. Nucl. Cardiol 2015;22(5):935-51.
Knuuti MJ, Nuutila P, Ruotsalainen U, et al. Euglycemic hyperinsulinemic clamp and oral glucose load in stimulating myocardial glucose utilization during positron emission tomography. J Nucl Med 1992;33(7):1255-62.
Lee BC, Moody JB, Poitrasson-Rivière A, et al. Blood pool and tissue phase patient motion effects on 82rubidium PET myocardial blood flow quantification. J Nucl Cardiol 2018;23:1-12.
Ficaro EP, Lee BC, Kritzman JN, Corbett JR. Corridor4DM: the Michigan method for quantitative nuclear cardiology. J Nucl Cardiol 2007;14(4):455-65.
Porenta G, Kuhle W, Czernin J, et al. Semiquantitative assessment of myocardial blood flow and viability using polar map displays of cardiac PET images. J Nucl Med 1992;33(9):1628-36.
FDG-PET/CT Technical Committee. FDG-PET/CT as an Imaging Biomarker Measuring Response to Cancer Therapy. Quantitative Imaging Biomarker Alliance, Version 1.05, Publicly Reviewed Version. QIBA; 2013. https://rsna.org/qiba. Accessed Feb 17, 2016.
Knuuti J, Schelbert HR, Bax JJ. The need for standardisation of cardiac FDG PET imaging in the evaluation of myocardial viability in patients with chronic ischaemic left ventricular dysfunction. Eur J Nucl Med Mol Imaging 2002;29(9):1257-66.
Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation 2002;105(4):539-42.
Moody JB, Murthy VL, Lee BC, Corbett JR, Ficaro EP. Variance estimation for myocardial blood flow by dynamic PET. IEEE Trans Med Imaging 2015;34(11):2343-53.
Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction. 2nd ed. New York: Springer; 2009.
Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol 2008;294(1):E15-26.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. http://www.R-project.org/.
Millman KJ, Aivazis M. Python for scientists and engineers. Comput Sci Eng 2011;13(2):9-12.
Paternostro G, Camici PG, Lammertsma AA, et al. Cardiac and skeletal muscle insulin resistance in patients with coronary heart disease. A study with positron emission tomography. J Clin Invest 1996;98(9):2094-9.
Nesterov SV, Deshayes E, Sciagrà R, et al. Quantification of myocardial blood flow in absolute terms using 82Rb PET imaging: results of RUBY-10 study. JACC Cardiovasc Imaging 2014;7(11):1119-27.
Klein R, Renaud JM, Ziadi MC, et al. Intra- and inter-operator repeatability of myocardial blood flow and myocardial flow reserve measurements using rubidium-82 PET and a highly automated analysis program. J Nucl Cardiol 2010;17:600-16.
Buck A, Wolpers HG, Hutchins GD, et al. Effect of carbon-11-acetate recirculation on estimates of myocardial oxygen consumption by PET. J Nucl Med 1991;32(10):1950-7.
Budinger TF, Yano Y, Huesman RH, et al. Positron emission tomography of the heart. Physiologist 1983;26(1):31-4.
Mullani NA, Goldstein RA, Gould KL, et al. Myocardial perfusion with rubidium-82. I. Measurement of extraction fraction and flow with external detectors. J Nucl Med 1983;24(10):898-906.
Lortie M, Beanlands RSB, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34(11):1765-74.
Prior JO, Allenbach G, Valenta I, et al. Quantification of myocardial blood flow with 82Rb positron emission tomography: clinical validation with 15O-water. Eur J Nucl Med Mol Imaging 2012;39(6):1037-47.
Schwaiger M, Pirich C. Reverse flow-metabolism mismatch: what does it mean? J Nucl Med 1999;40(9):1499-502.
Johnson NP, Sdringola S, Gould KL. Partial volume correction incorporating Rb-82 positron range for quantitative myocardial perfusion PET based on systolic-diastolic activity ratios and phantom measurements. J Nucl Cardiol 2011;18(2):247-58.
Renaud JM, Yip K, Guimond J, et al. Characterization of 3D PET systems for accurate quantification of myocardial blood flow. J Nucl Med 2017;58(1):103-9.
AlJaroudi W, Jaber WA, Grimm RA, Marwick T, Cerqueira MD. Alternative methods for the assessment of mechanical dyssynchrony using phase analysis of gated single photon emission computed tomography myocardial perfusion imaging. Int J Cardiovasc Imaging 2012;28(6):1385-94.
Acknowledgements
The authors thank Erin Pollock and Amanda Melvin for assistance with data collection.
Disclosure
J.B. Moody, B.C. Lee, and A. Poitrasson-Rivière are employees of INVIA. K.M. Hiller has nothing to declare. R.L. Weinberg has nothing to declare. E.P. Ficaro and J.R. Corbett are stockholders of INVIA, which produces 4DM, a clinical software package for cardiac PET analysis. V.L. Murthy declares research support from INVIA, LLC, and stock interest in General Electric, Mallinckrodt, Cardinal Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
JNC thanks Erick Alexanderson MD, Alondra Flores MD, Jessy Steve Masso MD, and Oscar Perez-Orpinel MD for providing the Spanish abstract; Zhuo He, Haipeng Tang MS, Min Zhao, and Weihua Zhou PhD for providing the Chinese abstract; and Jean-Luc Urbain, MD, PhD, CPE, Past President CANM, Chief Nuclear Medicine, Lebanon VAMC, PA for providing the French abstract.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moody, J.B., Hiller, K.M., Lee, B.C. et al. The utility of 82Rb PET for myocardial viability assessment: Comparison with perfusion-metabolism 82Rb-18F-FDG PET. J. Nucl. Cardiol. 26, 374–386 (2019). https://doi.org/10.1007/s12350-019-01615-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-019-01615-0