Abstract
Introduction
AURIGA is the largest real-world study to date to evaluate intravitreal aflibercept (IVT-AFL) in the treatment of diabetic macular edema (DME) or macular edema secondary to retinal vein occlusion in routine clinical practice. The 24-month outcomes in the DME cohort from across 11 participating countries are reported here.
Methods
AURIGA (NCT03161912) was a prospective observational study. The study enrolled eligible patients with DME for whom the decision to treat with IVT-AFL had previously been made by the attending physician. Patients were treated with IVT-AFL for up to 24 months at physician discretion according to local practice. The primary endpoint was mean change in visual acuity (VA; Early Treatment Diabetic Retinopathy Study [ETDRS] letters) from baseline to month 12 (M12). All statistical analyses were descriptive.
Results
In 1478 treatment-naïve and 384 previously treated patients with DME, the mean (95% confidence interval) change in VA from baseline was +6.7 (5.7, 7.6) and +7.4 (5.5, 9.4) letters by M12 and +5.9 (4.9, 6.9) and +8.1 (6.1, 10.1) letters by M24 (baseline [mean ± standard deviation]: 56.0 ± 19.8 and 50.8 ± 19.5 letters), respectively; 25.9% of treatment-naïve and 32.8% of previously treated patients achieved ≥ 15-letter gains by M24. The mean change in central retinal thickness from baseline to M24 was −110 (−119, −102) µm in treatment-naïve patients and −169 (−188, −151) µm in previously treated patients. By M6, M12, and M24, treatment-naïve patients had received 3.8 ± 1.7, 4.9 ± 2.8, and 5.7 ± 3.9 injections, respectively, and previously treated patients had received 3.9 ± 1.5, 4.9 ± 2.4, and 6.2 ± 3.6 injections, respectively. The safety profile of IVT-AFL was consistent with previous studies.
Conclusion
In AURIGA, treatment-naïve and previously treated patients with DME achieved clinically relevant functional and anatomic improvements following IVT-AFL treatment for up to 24 months in routine clinical practice. Even with the decreasing injection frequency observed, these gains were largely maintained throughout the study, suggesting long-term durability of the positive effects of IVT-AFL treatment. Infographic available for this article.
Trial Registration
ClinicalTrials.gov Identifier: NCT03161912 (May 19, 2017).
Infographic

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Diabetic macular edema (DME) is a leading cause of vision loss in patients with diabetes. |
Robust real-world data are needed on the long-term treatment effectiveness and safety of intravitreal aflibercept (IVT-AFL) in DME across a variety of clinical settings. |
The 24-month AURIGA study evaluated the effectiveness, treatment patterns, and safety of IVT-AFL in routine clinical practice across 11 countries in 1866 patients with DME. |
What was learned from the study? |
Clinically relevant functional and anatomic improvements were reported in both treatment-naïve and previously treated patients, and the safety profile of IVT-AFL was consistent with that of previous studies. |
These improvements were largely maintained across the study period, which may suggest long-term durability of the effects of IVT-AFL treatment; although greater visual acuity gains may have been achieved with more frequent treatment in line with label recommendations, other factors may have played a role. |
Digital Features
This article is published with digital features, including an infographic, to facilitate understanding of the article. To view digital features for this article go to: https://doi.org/10.6084/m9.figshare.24219940.
Introduction
Diabetic macular edema (DME) is a leading cause of vision loss in patients with diabetes, with approximately 5.5% of all patients clinically diagnosed with this retinal disease worldwide [1]. DME affects males more than females, although the severity of DME is generally greater in females [2]. The global prevalence of DME is rising, which is associated with the increasing prevalence of type 2 diabetes mellitus [3].
Anti-vascular endothelial growth factor (anti-VEGF) therapies constitute the first line of therapy [4]. These therapies target hyperpermeability of the retinal capillaries by inhibiting the upregulation of a number of cytokines, including VEGF and placental growth factor, to reduce permeability, lower levels of extracellular fluid, and thin the macula [3,4,5,6].
Intravitreal aflibercept (IVT-AFL) is an anti-VEGF agent [7] that was approved by the European Medicines Agency (EMA) [8] and US Food and Drug Administration (FDA) [9] for the treatment of visual impairment due to DME [10] following the 148-week VIVID and VISTA clinical trials [11,12,13].
Observational studies provide real-world evidence (RWE) that is complementary to data derived from randomized controlled trials (RCTs) on the effectiveness and long-term safety of therapies, treatment patterns, and disease burden and progression [14,15,16]. Real-world visual acuity (VA) gains following intravitreal anti-VEGF treatment for retinal disease are typically not as high as those observed in RCTs, which may be due to the overall lower injection frequency observed in routine clinical practice, in which a variety of factors may play a role [3].
AURIGA (NCT03161912) was a prospective observational study that assessed the long-term effectiveness, treatment patterns, patient-reported outcomes, and safety of IVT-AFL treatment in real-world settings. Here, we report the primary endpoint and final 24-month outcomes of AURIGA in treatment-naïve and previously treated patients with DME from 11 participating countries. AURIGA comprises the largest prospective real-world study of IVT-AFL to date in patients with DME, and the overall aim of the study was to generate global insights into opportunities for the optimization of DME management in clinical practice.
Methods
Study Design
AURIGA (NCT03161912) was a 24-month, prospective observational study conducted in 11 countries across 243 ophthalmology practices and eye clinics between November 24, 2017, and December 17, 2021. The study enrolled a total of 2529 treatment-naïve and previously treated patients to evaluate the effectiveness, treatment patterns, and safety of IVT-AFL in the management of DME and macular edema secondary to retinal vein occlusion in routine clinical practice. The initial decision to treat with IVT-AFL, as well as all retreatment and monitoring decisions, were made by the attending physician according to their local practice and marketing authorization (approval from a regulatory authority to market/sell a drug in a particular country or region). Sample size was calculated to enable sufficient precision in the assessment of the primary endpoint (mean change in VA from baseline to month 12) by country and by cohort, resulting in a planned enrollment of 1925 treatment-naïve and 825 previously treated patients with DME (see Supplementary Methods in the electronic supplementary material for details).
No master independent ethics committee (IEC) or institutional review board (IRB) approval was obtained, as no participating study site was deemed to be the main center for the study. Appendix I in the Supplementary Materials lists the local IRB/IEC committee names and approval numbers in all participating countries where relevant under local law. The AURIGA study was an observational study in which IVT-AFL was prescribed in the customary manner in accordance with the terms of the marketing authorization. There was no assignment of patients to a particular therapeutic strategy. All treatment decisions fell within current practice, and the prescription of IVT-AFL was clearly separate from the decision to include the patient in the study. No additional diagnostic or monitoring evaluations were required for participation in the study. Epidemiological methods were used for the analysis of the collected data.
The AURIGA study was conducted in accordance with the Helsinki Declaration of 1964. The applicable EMA guidelines and local laws and regulations in each country were adhered to. The recommendations of the European Federation of Pharmaceutical Industries and Associations (EFPIA), European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP), Good Pharmacovigilance Practices (GVP module VI), and the International Council for Harmonisation Guideline E3: Good Clinical Practice were also followed wherever possible. In each country where required, the protocol and any amendments thereof were reviewed and approved by the independent ethics committee or institutional review board of each study site. All patients provided written informed consent for participation in this study.
Patients and Procedures
All 11 countries participating in the AURIGA study contributed toward the overall DME cohort, namely Mainland China, Egypt, France, Germany, Italy, Kuwait, Lebanon, Russia, Saudi Arabia, Taiwan, and the United Arab Emirates. The key inclusion criteria were treatment-naïve and previously treated patients with DME, aged 18 years or older, for whom the decision to treat with IVT-AFL had already been made by the attending physician according to their local practice (see Supplementary Methods for list of exclusion criteria).
There were no prespecified treatment or retreatment criteria in this observational study, as the aim was to evaluate real-world treatment practices, effectiveness, and safety of IVT-AFL. Treatment and monitoring decisions were made at the discretion of the attending physician with consideration of the local IVT-AFL Summary of Product Characteristics (SmPC), and visual acuity and anatomic assessments were performed according to routine clinical practice at each study site.
Early Treatment Diabetic Retinopathy Study (ETDRS) charts were the preferred measure of best-corrected visual acuity (BCVA). Where ETDRS charts were unavailable, BCVA was assessed using other methods, including Snellen charts. In regions where BCVA was not part of the standard of care, conventional VA measurements were conducted and the data were later converted into ETDRS letter scores for statistical analysis [17]. Central retinal thickness (CRT) was measured by time-domain or spectral-domain optical coherence tomography (time-domain OCT/SD-OCT) using the instrument available at each site, and data generated by time-domain OCT were converted to SD-OCT measurements for later analysis [18]. The presence of retinal fluid was evaluated with SD-OCT and the data were interpreted at the study site.
Study Endpoints
The AURIGA study endpoints were assessed using data for each patient in the full analysis set (FAS) from visits nearest to month 6 (150–210 days after baseline), month 12 (300–420 days after baseline), and month 24 (660–794 days after baseline). Patients who received ≥ 1 IVT-AFL injection within the study period and who underwent ≥ 1 post-baseline observation were included in the FAS.
The primary endpoint was the mean change in VA from baseline to month 12. Secondary endpoints included the proportion of patients with prespecified VA gains and losses, mean change in CRT from baseline, mean number of injections received, mean time in study, and mean number of visits of each type. All endpoints were assessed at months 6, 12, and 24. In addition, VA and CRT data collected for the FAS throughout the study were analyzed at 4-weekly intervals (every 28 days) within a time window of +14/−13 days.
Further analyses included an exploratory analysis of the effect of IVT-AFL treatment on health-related quality of life, and a sensitivity analysis of the impact of the Coronavirus Disease 2019 (COVID-19) pandemic on study endpoints. For the COVID-19 sensitivity analysis, the “pre-COVID” group comprised patients who received their initial IVT-AFL treatment at least 360 days before the COVID-19 start date in their country of residence, whereas the “during COVID” group consisted of all other patients (see Supplementary Methods for further details).
Data from the study eye (defined as the first eye to receive IVT-AFL treatment) of each patient were used to evaluate the primary and secondary endpoints. Where treatment began simultaneously in both eyes, the eye with the worst VA at baseline was classified as the study eye.
Safety was monitored throughout the study, and the safety analysis set (SAS) included all patients who received ≥ 1 IVT-AFL injection during the study period. Ocular adverse events were reported for the study eye as well as the fellow eye in patients who received IVT-AFL treatment in both eyes. All treatment-emergent adverse events (TEAEs) were summarized using the Medical Dictionary for Regulatory Activities (MedDRA). Adverse events were defined as treatment-emergent if they began after the initial IVT-AFL injection or, at most, 30 days after the final injection.
Statistical Analysis
Statistical analyses were exploratory and descriptive in nature, as the study did not aim to confirm or reject predefined hypotheses. The data were analyzed descriptively using frequency distributions, percentages, and summary statistics per cohort and by country, as well as overall (pooled). For analyses of the mean change in VA and CRT from baseline, 95% confidence intervals (CI) were calculated, and any missing data were imputed using the last observation carried forward (LOCF) method; however, no baseline VA or CRT measurements were carried forward. No imputation was performed for the other endpoints. Statistical analyses were conducted with the Statistical Analysis System software v9.4 or higher (SAS Institute Inc., Cary, NC, USA).
Results
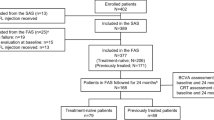
In total, 1516 treatment-naïve patients and 386 previously treated patients with DME were enrolled in the AURIGA study (Fig. 1). Treatment-naïve patients were enrolled from France, Germany, Italy, Russia, the Middle East, Taiwan, and Mainland China, whereas previously treated patients were enrolled from Italy, Russia, and Mainland China. The SAS comprised 97.6% of the treatment-naïve patients enrolled (1480/1516) and all 386 of the previously treated patients enrolled. The FAS consisted of 1478 treatment-naïve and 384 previously treated patients, after the exclusion of 38 treatment-naïve and two previously treated patients. Overall, 64.6% (n = 248) of previously treated patients in the FAS had switched to IVT-AFL due to persistent retinal fluid (intraretinal or subretinal fluid), followed by 19.5% (n = 75) who switched due to the recurrence of retinal fluid (Table S1).
In total, 62.7% (n = 926) and 32.4% (n = 479) of the treatment-naïve cohort completed the 12- and 24-month visits, respectively; in the previously treated cohort, 66.1% (n = 254) and 32.0% (n = 123) completed the 12- and 24-month visits, respectively.
The baseline demographics and disease characteristics of the patients in the FAS are listed in Table 1. Overall, patients were aged 22–92 years (mean age: treatment-naïve, 61.9; previously treated, 62.9), with over 75% of patients aged 55 years and over in each cohort. Mean VA and CRT at baseline was 56.0 letters and 437 μm for the treatment-naïve cohort, and 50.8 letters and 465 μm for the previously treated cohort, respectively. Median time from diagnosis to first IVT-AFL treatment was 0.9 months in the treatment-naïve cohort, and 15.7 months in the previously treated cohort. There were no marked differences among the participating countries in baseline demographics except for race (data not shown); however, there was some variation in time to treatment initiation (Table S2) and baseline VA and CRT (Table S3) among the different countries.
Functional and Anatomic Outcomes
At baseline, the treatment-naïve patients had a higher mean VA than the previously treated patients (56.0 ± 19.8 vs 50.8 ± 19.5 letters). The primary endpoint, mean (95% CI) change in VA from baseline to month 12, indicated an improvement in both treatment cohorts: +6.7 letters (5.7, 7.6) in the treatment-naïve cohort and +7.4 letters (5.5, 9.4) in the previously treated cohort. The primary outcome stratified by gender is provided in Table S4.
Improved VA was reported within the first 6 months of IVT-AFL treatment in both cohorts, and these gains were generally maintained across the 24-month study (Figs. 2 and 3; Table S5). Letter gains of +7.3 at month 6, +6.7 at month 12, and +5.9 at month 24 were reported in treatment-naïve patients; and letter gains of +8.7 at month 6, +7.4 at month 12, and +8.1 at month 24 were reported in previously treated patients. Keeping in consideration that the mean baseline VA varied from 48.5 to 67.1 letters among the seven participating countries/regions, the greatest VA gains in treatment-naïve patients were observed in Taiwan, and the lowest VA gains were reported in Germany and Italy (Fig. 2). Among the three countries contributing to the previously treated cohort, the greatest VA gains were observed in patients from Russia.
Change in mean VA from baseline to months 6, 12, and 24 in each country and CRT over 24 months in a treatment-naïve and b previously treated patients with diabetic macular edema who were treated with intravitreal aflibercept in routine clinical practice. BL, baseline; CRT, central retinal thickness; VA, visual acuity; W, week
When mean change in VA was stratified by baseline VA (< 35 letters, 35–69 letters, and ≥ 70 letters), the greatest gains by month 12 were observed in patients with the lowest baseline VA, with +26.5 and +25.4 letters achieved in the treatment-naïve and previously treated cohorts, respectively (Fig. 4). In contrast, treatment-naïve and previously treated patients with the highest baseline VA experienced VA losses of −0.1 and −1.3 letters by month 12, respectively. A similar trend was observed in the mean change in VA by month 24.
The proportions of treatment-naïve patients who achieved ≥ 5-letter, ≥ 10-letter, and ≥ 15-letter VA gains by month 24 were 55.6%, 37.7%, and 25.9%, respectively; in previously treated patients, these proportions were 60.6%, 44.4%, and 32.8%, respectively (Figure S1). The proportions of treatment-naïve and previously treated patients who maintained vision over the 24-month study (i.e., lost fewer than 15 letters) were 90.1% and 91.1%, respectively.
In AURIGA, the proportions of patients who achieved ≥ 70 letters by months 12 and 24 were 43.9% (649/1478) and 43.0% (635/1478) in treatment-naïve patients, and 37.8% (145/384) and 42.4% (163/384) in previously treated patients. Of patients with a baseline VA of ≥ 70 letters, 73.7% (337/457) of treatment-naïve patients and 77.5% (62/80) of previously treated patients had maintained a VA of ≥ 70 letters by month 24.
Surprisingly, the baseline CRT of the previously treated cohort (465 ± 148 µm [n = 360]) was slightly higher than that of the treatment-naïve cohort (437 ± 140 µm [n = 1292]) (Table S3). A robust rapid reduction in the mean CRT was observed within the first 6 months of treatment in both cohorts (Figs. 2 and 3), with a reported mean (95% CI) change from baseline of −117 (−125, −109) µm for the treatment-naïve cohort and −175 (−193, −157) µm for the previously treated cohort. These reductions in CRT were maintained throughout the study, with a mean change from baseline of −110 (−119, −102) µm for the treatment-naïve cohort and −169 (−188, −151) µm for the previously treated cohort by month 24. At each time point, the decrease in CRT was higher in the previously treated cohort than in the treatment-naïve cohort.
Treatment Pattern
The mean ± standard deviation (SD) number of IVT-AFL treatments received by each treatment cohort by months 6, 12, and 24 was similar across the two cohorts: 3.8 ± 1.7, 4.9 ± 2.8, and 5.7 ± 3.9, respectively, in treatment-naïve patients and 3.9 ± 1.5, 4.9 ± 2.4, and 6.2 ± 3.6, respectively, in previously treated patients. Country-specific differences in the mean number of IVT-AFL treatments were noted, with treatment-naïve patients in France and Germany receiving the highest number of injections by each time point (Fig. 5 and Table S6). A low number of injections was received by patients in China due to their later study start date (only 31.7% and 5.4% of patients in China were able to complete the 12- and 24-month visits across both cohorts). The majority of patients (86.0%) in the overall treatment-naïve group did not receive all five of the initial monthly doses recommended as per the IVT-AFL product label. The mean ± SD time in the study was 16.9 ± 6.5 months in the treatment-naïve cohort and 17.9 ± 5.5 months in the previously treated cohort, with some differences observed among the participating countries (China had the lowest mean time in study [13.2 months], whereas Russia had the greatest [21.2 months]).
The main reasons for end of observation before study closure included loss to follow-up (25.3% [374/1478] and 31.5% [121/384]) and switch to another therapy (8.5% [126/1478] and 3.4% [13/384]) in the treatment-naïve and previously treated cohorts, respectively (Table 2).
The last completed treatment interval was ≥ 12 weeks in 34.1% (121/355) and 26.7% (352/1317) of patients at month 12, and 38.6% (137/355) and 32.4% (427/1317) of patients at month 24 in the previously treated and treatment-naïve cohorts, respectively.
Impact of COVID-19 Pandemic
Patients in both cohorts treated during the COVID-19 pandemic had similar functional and anatomic outcomes by month 12 and month 24 and received a similar number of injections compared with those treated prior to the pandemic (data not shown).
Patient-Reported Outcomes
In AURIGA, the patient-reported outcomes (PROs) assessed comprised the National Eye Institute Visual Function Questionnaire 25 (NEI VFQ-25) [19], Falls Efficacy Scale International (FES-I) [20], and Hospital Anxiety and Depression Scale (HADS) [21]. In addition, indirect costs and resource use were evaluated with the Costs and Outcomes of Retinal Disease (COMETA) questionnaire developed by Bayer AG (Appendix II).
Participation in the PRO questionnaires was voluntary. Only a small proportion of patients contributed, and the number of responses to the questionnaires decreased markedly between baseline, month 12, and month 24. This prevented the completion of some of the analyses. The majority of data were collected for the NEI VFQ-25, which are reported in Table 3. There was a clinically relevant improvement in the patients’ vision-related quality of life (i.e., ≥ 4-point change [22, 23]) from baseline to month 12 of 5.2 ± 13.9 and 5.2 ± 12.9 points in both the treatment-naïve and previously treated cohorts, respectively. By month 24, patients had maintained a clinically relevant improvement in this outcome versus baseline (4.1 ± 15.4 [treatment-naïve] and 5.7 ± 17.2 [previously treated] points).
Safety
In the SAS for the treatment-naïve and previously treated cohorts, 19.7% (291/1480) and 3.1% (12/386) of patients, respectively, experienced TEAEs (Table 4), with ocular TEAEs occurring in 13.1% (194/1480) and 1.3% (5/386) of patients, respectively. The most common ocular TEAEs across both cohorts were conjunctival hemorrhage (1.5% [28/1866]), cataract (1.4% [26/1866]), and epiretinal membrane (1.1% [21/1866]).
In terms of intraocular inflammation, one case each of endophthalmitis, eye infection, hypopyon, and vitreous fibrin was reported in the treatment-naïve cohort, none of which were considered serious; there was also a case of uveitis in this cohort, which was considered both serious and study drug-related. One case of endophthalmitis was reported in the previously treated cohort, and this was considered both serious and study drug-related. The treatment-naïve cohort had one case each of retinal vascular occlusion and retinal vein occlusion, neither of which were considered serious. There were no cases of retinal vasculitis or retinal neovascularization in either cohort.
Serious TEAEs occurred in 4.8% (71/1480) and 1.3% (5/386) of patients in the treatment-naïve and previously treated cohorts, respectively (Table 4). Retinal detachment was the most common serious ocular TEAE (0.1% [2/1866]), with the remaining serious ocular TEAEs occurring in one patient each (all in treatment-naïve patients). The most common serious non-ocular TEAEs were cerebrovascular accident (0.4% [6/1480]) and myocardial infarction (0.3% [4/1480]) in the treatment-naïve cohort, and transient ischemic attack (0.5% [2/386]) in the previously treated cohort.
There were no discontinuations of IVT-AFL treatment due to TEAEs in the previously treated cohort. In the treatment-naïve cohort, three patients discontinued the study due to TEAEs, one of which was considered study drug-related and serious (cerebrovascular accident); the remaining TEAEs were altered state of consciousness, pregnancy, and dyspnea.
Four deaths were reported during the study, none of which were deemed to be study drug-related: three in the treatment-naïve cohort and one in the previously treated cohort. Deaths were due to coma, an unknown cause, and liver cirrhosis in the treatment-naïve cohort (n = 1 each), and as a complication of diabetes mellitus in the previously treated cohort (n = 1).
Discussion
In AURIGA, the real-world effectiveness, treatment patterns, and safety of IVT-AFL were assessed in patients with DME enrolled from across 11 countries. Here, we reported the primary endpoint and final, 24-month results from the AURIGA DME cohort, which consisted of patients from France, Germany, Italy, Mainland China, the Middle East, Russia, and Taiwan.
From baseline to month 12, the mean change in VA was +6.7 letters in the treatment-naïve cohort and +7.4 letters in the previously treated cohort following a mean of 4.9 injections in both. By month 24, the mean change in VA decreased slightly in treatment-naïve patients to +5.9 letters after a mean of 5.7 injections, and increased slightly in previously treated patients to +8.1 letters after a mean of 6.2 injections. Patients with a lower mean baseline VA achieved the greatest gains, whereas the opposite trend was observed for patients with a higher mean baseline VA. In both the treatment-naïve and previously treated cohorts, a robust decrease in CRT was observed at month 6 and was maintained through months 12 and 24. At each time point, the decrease in CRT was numerically higher in the previously treated cohort than in the treatment-naïve cohort; by month 24, the mean change in CRT from baseline was −110 µm in treatment-naïve patients and −169 µm in previously treated patients. Overall, the mean time in the study was 16.9 months in the treatment-naïve cohort and 17.9 months in the previously treated cohort.
The functional and anatomic improvements achieved in patients with DME in AURIGA were similar to or greater than the gains observed in other prospective real-world studies of IVT-AFL. In the APOLLON study conducted in France, treatment-naïve and previously treated patients gained +6.5 letters overall between baseline and month 12 following 7.6 IVT-AFL injections (n = 147) [24]; this decreased to +3.9 letters by month 24 after a mean total of 11.6 injections (n = 168) [25]. From baseline to month 12, the reduction in CRT in APOLLON ranged from 130 to 138 µm across the two treatment cohorts. In the DRAKO study in the United Kingdom, treatment-naïve patients (n = 488) gained +1.9 letters by month 12 after 6.3 IVT-AFL injections [26].
Gains in VA in real-world studies are typically lower than those observed in clinical trials [3]; in AURIGA, the VA gains were lower than those reported in key RCTs of IVT-AFL in DME. In the VIVID and VISTA studies, patients receiving IVT-AFL were administered 2 mg every 4 weeks (2q4) or 2 mg every 8 weeks (2q8) [11]. From baseline to week 52 in the 2q4 and 2q8 cohorts, patients in VIVID gained +10.5 and +10.7 letters, respectively, and patients in VISTA gained +12.5 and +10.7 letters, respectively, with a mean reduction in CRT that ranged from 183 to 195 µm across the four IVT-AFL cohorts. The gains achieved by week 52 in these RCTs were maintained to week 100 [12] and week 148 [13]. In VIVID and VISTA, the mean number of IVT-AFL injections received by week 52 ranged from 8.4 to 12.2 [11], which was notably higher than that received by month 12 in the AURIGA cohorts. Further, in the Protocol T trial, patients receiving IVT-AFL achieved +13.3 and +12.8 letters after 1 and 2 years of treatment, respectively, following a median of 9 and 15 injections [27, 28].
In a recent systematic review and meta-analysis that assessed the effectiveness of IVT-AFL using literature published up until February 2020, a pooled analysis of VA across 18 eligible studies (6 RCTs and 12 RWE studies) reported a +9.3-letter improvement by month 12; a similar analysis across two RCTs and two RWE studies indicated a gain of +6.8 letters by month 24 [29]. For comparison, IVT-AFL treatment yielded slightly higher gains in VA by month 12 than with ranibizumab based on a pooled analysis of the six studies that included comparator arms (weighted mean difference of +1.76 letters; 95% CI 0.75–2.76; P = 0.001).
Overall, these findings suggest that despite the low injection frequency in AURIGA, relatively robust gains in VA were achieved and were generally maintained in patients treated for DME with IVT-AFL in a real-world setting. Greater gains may have been achieved in these patients with more frequent IVT-AFL treatment, particularly within the first year, given that a mean of 4.9 injections were received and 86.0% of patients in the treatment-naïve group did not receive all five of the recommended initial monthly doses [8, 9]. This treatment pattern was observed across most of the participating countries, with only France and Germany reporting a mean number of injections close to the maximum of five and eight possible treatments within the first 6 months and first year, respectively, in treatment-naïve patients. This is an important avenue for further research, as differences in reimbursement, access, adherence, and clinical practice between countries may ultimately have a marked effect on patient outcomes.
The safety profile of IVT-AFL was consistent with previous studies [13, 25,26,27]. There was one case each of uveitis and endophthalmitis that were considered both serious and treatment-related. There were no cases of retinal vasculitis or retinal neovascularization reported.
The strengths of the AURIGA study include the prospective design, the large number of patients enrolled, and the large number of participating centers across 11 countries. This facilitated the collection of robust data from heterogeneous patient cohorts and real-world clinical settings, and these data were analyzed both overall and stratified per country. As the underlying disease cannot be cured after 12 months of treatment, additional data from long-term follow-up in studies, such as AURIGA, deliver valuable information on drug effectiveness, disease progression, and treatment patterns in the management of DME [15]. Indeed, RWE is in increasing demand by regulators and healthcare providers, as these studies generate valuable information on aspects that RCTs are not designed to evaluate, and more measures are being put in place to ensure rigorous data collection and mitigate bias [14,15,16].
There are several limitations inherent in the observational nature of the AURIGA study, given that the aim was to assess IVT-AFL treatment outcomes in routine clinical practice. In contrast with RCTs, real-world treatment and monitoring schedules are at the discretion of the attending physician; while treatment decisions will have been made based on clinical experience in managing the care of patients with DME, results can be highly variable. This can lead to missing data that may limit the interpretation of study findings. Specifically in AURIGA, the month 24 data from Mainland China must be interpreted with caution, as patient enrollment was delayed compared with the other countries, and most patients were unable to complete the month 24 visit. Fewer countries committed to enrolling previously treated than treatment-naïve patients, and the exclusion criteria of the study reduced the eligible patient population who could be enrolled in the previously treated cohort (i.e., patients could only have been enrolled in this cohort if they had received prior treatment with steroids or intravitreal anti-VEGF agents other than IVT-AFL). Thus, the previously treated cohort is smaller than the treatment-naïve cohort. In addition, error and information bias may have been introduced as a result of the various methodologies used in AURIGA to assess VA and CRT across different real-world settings [30,31,32].
Conclusions
The AURIGA study is the largest prospective observational study to date on the treatment of DME with IVT-AFL and was conducted across 11 countries in a variety of real-world settings. Even with the relatively low treatment frequency observed compared with RCTs, clinically relevant functional and anatomic improvements were reported for both treatment-naïve and previously treated patients, and these gains were largely maintained throughout the 24-month study. These findings suggest long-term durability of the effects of IVT-AFL treatment, although greater gains may have been achieved with more frequent treatment in line with the recommendations on the product label.
Data Availability
Availability of the data underlying this publication will be determined later according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing.” This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 1, 2014.
Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal.
Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
References
Im JHB, Jin YP, Chow R, Yan P. Prevalence of diabetic macular edema based on optical coherence tomography in people with diabetes: a systematic review and meta-analysis. Surv Ophthalmol. 2022;67(4):1244–51.
Schiefelbein J, Müller M, Kern C, Herold T, Liegl R, Fasler K, et al. Gender-related differences in patients treated with intravitreal anti-vascular endothelial growth factor medication for diabetic macular oedema. Eur J Ophthalmol. 2020;30(6):1410–7.
Browning DJ, Stewart MW, Lee C. Diabetic macular edema: evidence-based management. Indian J Ophthalmol. 2018;66(12):1736–50.
Zhang J, Zhang J, Zhang C, Zhang J, Gu L, Luo D, et al. Diabetic macular edema: current understanding, molecular mechanisms and therapeutic implications. Cells. 2022;11(21):3362.
Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, et al. Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res. 2018;63:20–68.
Uemura A, Fruttiger M, D’Amore PA, De Falco S, Joussen AM, Sennlaub F, et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog Retin Eye Res. 2021;84: 100954.
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171–85.
European Medicines Agency. Eylea Summary of product characteristics 2012 [updated January 2023; cited May 2023]. Available from: https://www.ema.europa.eu/en/documents/product-information/eylea-epar-product-information_en.pdf.
Food and Drug Administration. Eylea prescribing information 2011 [updated September 2022; cited May 2023]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125387s076lbl.pdf.
Regeneron Pharmaceuticals, Inc. Eylea® (aflibercept) injection receives EU approval for the treatment of diabetic macular edema (DME) [reviewed August 2014; cited May 2023]. Available from: https://investor.regeneron.com/news-releases/news-release-details/eylear-aflibercept-injection-receives-eu-approval-treatment/.
Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–54.
Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122(10):2044–52.
Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do DV, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123(11):2376–85.
Burns L, Roux NL, Kalesnik-Orszulak R, Christian J, Hukkelhoven M, Rockhold F, et al. Real-world evidence for regulatory decision-making: guidance from around the world. Clin Ther. 2022;44(3):420–37.
Daien V, Eldem BM, Talks JS, Korobelnik JF, Mitchell P, Finger RP, et al. Real-world data in retinal diseases treated with anti-vascular endothelial growth factor (anti-VEGF) therapy—a systematic approach to identify and characterize data sources. BMC Ophthalmol. 2019;19(1):206.
Klonoff DC. The new FDA real-world evidence program to support development of drugs and biologics. J Diabetes Sci Technol. 2020;14(2):345–9.
Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing Snellen visual acuity measurements. Retina. 2010;30(7):1046–50.
Diabetic Retinopathy Clinical Research Network Writing Committee, Bressler SB, Edwards AR, Chalam KV, Bressler NM, Glassman AR, et al. Reproducibility of spectral-domain optical coherence tomography retinal thickness measurements and conversion to equivalent time-domain metrics in diabetic macular edema. JAMA Ophthalmol. 2014;132(9):1113–22.
Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, et al. Development of the 25-item national eye institute visual function questionnaire. Arch Ophthalmol. 2001;119(7):1050–8.
Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing. 2005;34(6):614–9.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Submacular Surgery Trials Research Group. Evaluation of minimum clinically meaningful changes in scores on the National Eye Institute Visual Function Questionnaire (NEI-VFQ) SST Report Number 19. Ophthalmic Epidemiol. 2007;14(4):205–15.
Suñer IJ, Kokame GT, Yu E, Ward J, Dolan C, Bressler NM. Responsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trials. Invest Ophthalmol Vis Sci. 2009;50(8):3629–35.
Korobelnik J, Daien V, Faure C, Tadayoni R, Giocanti-Aurégan A, Dot C, et al. Real-world outcomes following 12 months of intravitreal aflibercept monotherapy in patients with diabetic macular edema in France: results from the APOLLON study. Graefes Arch Clin Exp Ophthalmol. 2020;258(3):521–8.
Korobelnik JF, Daien V, Faure C, Tadayoni R, Giocanti-Aurégan A, Dot C, et al. Two-year outcomes of the APOLLON observational study of intravitreal aflibercept monotherapy in France in patients with diabetic macular edema. Sci Rep. 2022;12(1):18242.
Sivaprasad S, Ghanchi F, Kelly SP, Kotagiri A, Talks J, Scanlon P, et al. Evaluation of standard of care intravitreal aflibercept treatment of diabetic macular oedema treatment-naive patients in the UK: DRAKO study 12-month outcomes. Eye (Lond). 2022;36(1):64–71.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: 2-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351–9.
Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–203.
Santhakumaran S, Salimi A, Brunetti VC, Galic J. Efficacy and safety of aflibercept therapy for diabetic macular edema: a systematic review and meta-analysis. J Curr Ophthalmol. 2022;34(2):133–47.
Lovie-Kitchin JE. Is it time to confine Snellen charts to the annals of history? Ophthalmic Physiol Opt. 2015;35(6):631–6.
Finger RP, Daien V, Talks JS, Mitchell P, Wong TY, Sakamoto T, et al. A novel tool to assess the quality of RWE to guide the management of retinal disease. Acta Ophthalmol. 2021;99(6):604–10.
Finger RP, Sakamoto T, Talks J, Daien V, Wong T, Eldem B, et al. Navigating real-world evidence in ophthalmology: Modern Retina; 2021 [5 January 2021; cited May 2023]. Available from: https://www.modernretina.com/view/navigating-real-world-evidence-in-ophthalmology.
Acknowledgements
The authors thank all the patients and clinical investigators who participated in the AURIGA study. Appendix III in the Supplementary Materials provides the list of AURIGA lead study investigators in each of the participating countries. The AURIGA study was sponsored by Bayer AG, Leverkusen, Germany.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial support for the preparation of this manuscript, under the guidance of the authors, was provided by Afsaneh Khetrapal and Natasha Beeton-Kempen of ApotheCom, UK, and was funded by Bayer Consumer Care AG, Basel, Switzerland, in accordance with Good Publication Practice (GPP) standards (Ann Intern Med 2022;175:1298–1304).
Funding
The AURIGA study was sponsored by Bayer AG, Leverkusen, German, and the Rapid Service Fee was funded by Bayer Consumer Care AG, Basel, Switzerland.
Author information
Authors and Affiliations
Consortia
Contributions
Helmut Allmeier, Tobias Machewitz, and Kristian J. Johnson were responsible for and/or involved in study conception and design; Simone Donati, Chang-Hao Yang, Xun Xu, Marco Mura, Audrey Giocanti-Aurégan, Hans Hoerauf, Helmut Allmeier, Tobias Machewitz, Kristian T. Johnson, and Elina Santoro were responsible for and/or involved in data collection and interpretation. Tobias Machewitz was responsible for statistical analyses. All authors contributed toward drafting the manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Simone Donati declares that he has no competing interests. Chang-Hao Yang has received consulting fees and grants from Allergan, Bayer, and Novartis. Xun Xu declares that he has no competing interests. Marco Mura declares that he has no competing interests. Audrey Giocanti-Aurégan has acted as a clinical investigator for Bayer and received consulting fees from AbbVie, Alcon, Bayer, Novartis, Roche, and Théa. Hans Hoerauf has received consulting fees from AbbVie, Alcon, Allergan, Bayer, Heidelberg Engineering, Novartis, Oxurion, and Roche. Helmut Allmeier is an employee of Bayer Consumer Care AG, Basel, Switzerland. Tobias Machewitz is an employee of Bayer AG, Berlin, Germany. Kristian T. Johnson is an employee of Bayer U.S. LLC, Cambridge, MA, USA. Elina Santoro has received consulting fees and grants from Alcon, Bayer, and Novartis.
Ethical Approval
No master Independent Ethics Committee (IEC) or Institutional Review Board (IRB) approval was obtained as no participating study site was deemed to be the main center for the study. Appendix I in the Supplementary Materials lists the local IRB/IEC committee names and approval numbers in all participating countries where relevant under local law. The AURIGA study was an observational study in which IVT-AFL was prescribed in the customary manner in accordance with the terms of the marketing authorization. There was no assignment of patients to a particular therapeutic strategy. All treatment decisions fell within current practice, and the prescription of IVT-AFL was clearly separated from the decision to include the patient in the study. No additional diagnostic or monitoring evaluations were required for participation in the study. Epidemiological methods were used for the analysis of the collected data.
The AURIGA study was conducted in accordance with the Helsinki Declaration of 1964. The applicable EMA guidelines and local laws and regulations in each country were adhered to. The recommendations of the European Federation of Pharmaceutical Industries and Associations (EFPIA), European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP), Good Pharmacovigilance Practices (GVP module VI), and the International Council for Harmonisation Guideline E3: Good Clinical Practice were also followed wherever possible. In each country where required, the protocol and any amendments thereof were reviewed and approved by the independent ethics committee or institutional review board of each study site. All patients provided written informed consent for participation in this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Donati, S., Yang, CH., Xu, X. et al. Intravitreal Aflibercept for the Treatment of Diabetic Macular Edema in Routine Clinical Practice: Results from the 24-Month AURIGA Observational Study. Ophthalmol Ther 13, 161–178 (2024). https://doi.org/10.1007/s40123-023-00829-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00829-3