Abstract
Introduction
To assess the relationship between low bone mineral density (BMD), anti-cyclic citrullinated peptide-2 (anti-CCP2) antibodies, and disease activity in patients with established rheumatoid arthritis (RA).
Methods
Patients enrolled in a single-center, observational cohort registry of patients with RA. Eligible patients had known BMD, as measured by digital X-ray radiogrammetry (DXR–BMD), and anti-CCP2 antibody measurements at the same time point or within 6 months. Anti-CCP2–immunoglobulin (Ig)G-positive (+) patients (≥ 20 U/mL) were distributed into three equal groups (Gp1–3), representing increasing anti-CCP2 antibody concentrations. Associations between BMD and anti-CCP2 antibody status and titer were explored in multivariate regression analyses controlling for covariates (including age, duration of RA, use of steroids, use of osteoporosis medication). Association between disease activity (DAS28 [CRP] < 2.6) and bone loss was also explored.
Results
A total of 149 patients (all women) were included (47 anti-CCP2 antibody negative [−], 102 anti-CCP2+ [34\titer group]). Mean disease duration was greater in the three anti-CCP2+ groups vs. the anti-CCP2− group. DXR–BMD was lower in the anti-CCP2+ vs. the anti-CCP2− groups (Gp1–3 vs. anti-CCP2−: P < 0.0001 for left and right hands). DXR–BMD decreased with increasing anti-CCP2 titer (P < 0.001 for left and right hands). Patients with low DXR–BMD were less likely to have a DAS28 (CRP) < 2.6 (P = 0.0181).
Conclusion
Among patients with established RA, data suggest that anti-CCP2+ patients, particularly those with high anti-CCP2 antibody titers, have lower hand BMD, and patients with lower hand BMD are less likely to have low disease activity.
Funding
Bristol-Myers Squibb.
Trial Registration
Clinicaltrials.gov identifier, NCT01793103.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is associated with bone loss, erosions, and osteoporosis [1,2,3]. Several studies suggest that both erosive RA and osteoporosis share a common cellular pathway, which involves inflammatory activation of osteoclasts and decreased osteoblast activation [4, 5]. Low bone mineral density (BMD) in patients with RA increases the risk of fractures and overall mortality, especially in postmenopausal women [6,7,8]. Hand BMD loss, as measured by digital X-ray radiogrammetry (DXR)—a sensitive quantitative method for detecting early bone loss by measuring the cortical bone of metacarpal diaphysis—is an independent predictor of radiographic joint damage progression, including erosions [9,10,11].
A comparison of DXR and dual-energy X-ray absorptiometry (DXA) revealed that DXR appears to be more sensitive than DXA in detecting early bone loss in patients with RA [9]. Several studies have demonstrated a treatment effect of conventional and biologic disease-modifying antirheumatic drugs (bDMARDs) on BMD loss using DXR [4, 12,13,14,15,16,17]; however, data are limited on identifying factors that are associated with BMD loss. Given the correlation of DXR–BMD with increased fracture risk and mortality [7, 8], it would be beneficial to identify a reliable prognostic factor that is associated with hand BMD loss and treatment outcomes in patients with RA. The identification of such prognostic factors could assist rheumatologists in identifying patients at risk of radiographic progression and inform treatment decisions, with the aim of preventing bone erosion.
Anti-citrullinated protein antibody (ACPA) positivity is associated with poor prognosis in RA, and testing for ACPA has become standard practice in the diagnosis of RA [18, 19]. Recent studies have suggested that ACPA can stimulate bone loss by inducing the differentiation of precursors into bone-resorbing osteoclasts [20, 21]. In patients who are positive for anti-cyclic citrullinated peptide-2 (anti-CCP2, a surrogate of ACPA) antibodies, structural bone damage can start before the clinical onset of RA [22]. Elevated anti-CCP2 antibody levels have been found to be independent predictors of localized hand DXR–BMD loss in patients with early RA [23]. Furthermore, analysis of data from the Pavia Early Arthritis Clinic, a single-center cohort of patients, showed that anti-CCP2 antibodies and rheumatoid factor were associated with systemic bone loss in patients with early, untreated RA [24]. However, the relationship between hand BMD loss and anti-CCP2 antibodies in patients with established RA is unclear. Data from a recent single-center population study using DXA–BMD showed a negative, titer-dependent effect of ACPA on systemic bone mass at femoral sites in patients with established RA [5]. This analysis was performed to assess the association between hand DXR–BMD and anti-CCP2 antibody status, DXR–BMD and anti-CCP2 titer, as well as DXR–BMD and RA disease activity among patients with established RA.
Methods
Study Population
The Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS; ClinicalTrials.gov identifier NCT01793103) registry was initiated in 2003. Details regarding the design of the registry have been reported previously [25,26,27]. BRASS is a single-center, prospective, observational, longitudinal cohort of more than 1400 adults with established or recent-onset RA who are being followed in a hospital-based practice of 21 rheumatologists in Boston, Massachusetts. The BRASS Registry has been conducted in accordance with International Society for Pharmacoepidemiology Guidelines for Good Pharmacoepidemiology Practices, applicable regulatory requirements, and ethical tenets originating in the Declaration of Helsinki. The study protocol and informed consent document were reviewed and approved by the Brigham and Women’s Hospital Institutional Review Board (approval number 2002P001763). All patients provided written informed consent before participating in the BRASS Registry. The present study population represents a subset of the BRASS cohort, and eligible patients had DXR–BMD and anti-CCP2 antibody measurements at the same time point or within 6 months.
Measures and Data Collection
Patient demographic data and clinical characteristics, disease activity, and laboratory parameters were assessed at baseline and annually thereafter. Digitized hand radiographs were collected at baseline and at 2, 5, 7, 10, and 12 years and will be collected to at least 15 years (Fig. 1). Hand BMD was measured at the metacarpal bones of the second, third, and fourth digits using DXR–BMD (DXR-online, Sectra Imtec AB, Linköping, Sweden) as described previously [28]. Anti-CCP2 antibody level was measured using a validated ELISA (Inova Diagnostics, San Diego, California, USA until its discontinuation in 2011; thereafter Euro-Diagnostica [distributed by IBL-America, Minneapolis, Minnesota, USA]). Patient-reported outcomes were assessed with a follow-up questionnaire every 6 months (Fig. 1).
Study Outcomes
Patient demographic data and clinical characteristics were reported by anti-CCP2 antibody status (anti-CCP2 positive [+] and anti-CCP2 negative [−]), and anti-CCP2 antibody titer group (Group [Gp] 1–3). Anti-CCP2 antibody status was defined either as anti-CCP2+ (≥ 20 U/mL) or anti-CCP2− (< 20 U/mL). Anti-CCP2+ patients were divided equally into three subgroups based on the tertiles of anti-CCP2 antibody titers as Gp1, 20–96.6 U/mL; Gp2, 96.7–309.6 U/mL, and Gp3, 309.7–580 U/mL. Mean DXR–BMD was reported by anti-CCP2 antibody status and titer groups. The association between Disease Activity Score in 28 joints (DAS28) (C-reactive protein [CRP]) < 2.6 and bone loss was analyzed in patients with DXR–BMD < 0.5 g/cm2 (left or right hand) vs. ≥ 0.5 g/cm2 (both hands).
Statistical Analysis
A cross-sectional analysis was performed on available data for DXR–BMD and anti-CCP2 antibody level measured within 6 months of the DXR–BMD measurement. For descriptive statistics, Wilcoxon rank-sum test (or Kruskal–Wallis test) was used for continuous variables and Pearson’s Chi-square test for categorical variables. Associations between DXR–BMD (left, right, and combined [average of left and right hands]) and anti-CCP2 antibody status and titer (Gp1–3) were explored in multivariate analyses using linear regression controlling for covariates of age, duration of RA, body mass index (BMI), DAS28 (CRP), smoking status, use of steroids, bDMARDs, and osteoporosis medication. With DXR–BMD as the dependent variable, we explored anti-CCP2 antibody level as a continuous variable (linear trend) in relation to DXR–BMD, and explored anti-CCP2 antibody status as a categorical variable and included different anti-CCP2 antibody groups as reference groups.
Associations between DXR–BMD and DAS28 (CRP) < 2.6 in patients with DXR–BMD ≥ 0.5 and < 0.5 g/cm2 were explored using a logistic model controlling for covariates of age, duration of RA, BMI, smoking status, use of steroids, bDMARDs, and osteoporosis medication.
Results
Patient Disposition and Patient Characteristics by Anti-CCP2 Antibody Status and Titer Group
A total of 149 patients (all postmenopausal women) had an anti-CCP2 antibody measurement within 6 months of a DXR–BMD measurement: 47 (31.5%) were anti-CCP2−; 102 (68.5%) were anti-CCP2+. Sample sizes for the left and right hands were similar. Of the 102 patients with anti-CCP2+ status, 34 were included in each titer group (Gp1, Gp2, and Gp3). Patient characteristics by anti-CCP2 antibody status and titer group are shown in Table 1. Age, BMI, DAS28 (CRP), smoking status, use of steroids, bDMARDs, and osteoporosis medication did not differ significantly by anti-CCP2 antibody status (±) or between groups (Table 1). Mean duration of RA was different between the groups (P < 0.05); a longer duration of RA was also reported in anti-CCP2+ patients vs. anti-CCP2− patients (Table 1; P < 0.05). However, there was no clear pattern of disease duration between anti-CCP2+ titer groups.
DXR–BMD by Anti-CCP2 Antibody Titer Group
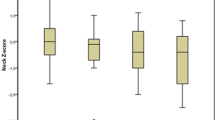
In the univariate analysis, DXR–BMD was lower in the anti-CCP2+ group vs. the anti-CCP2− titer groups (Gp1–3 vs. anti-CCP2−: P < 0.0001 for left and right hands). DXR–BMD decreased with increasing anti-CCP2 antibody titer for the left hand (mean [SD]; anti-CCP2− group, 0.56 [0.08]; Gp1, 0.51 [0.09]; Gp2, 0.51 [0.08]; Gp3, 0.48 [0.1]), and right hand (0.58 [0.08]; 0.52 [0.09]; 0.52 [0.08]; 0.49 [0.1], respectively) (Fig. 2).
Association between DXR–BMD and anti-CCP2 antibody status and titer in a left hand and b right hand. Number of patients in each titer group: anti-CCP2–, n = 47; Gp1, n = 34; GP2, n = 34; Gp3, n = 34. Timeframe between DXR–BMD and anti-CCP2 measurements (months [SD]) were 0.6 (1.4) for anti-CCP2–, 1.8 (2.3) for Gp1, 1.1 (1.8) for GP2, and 1.0 (1.7) for Gp3 (P > 0.05 for comparison between the anti-CCP2+ groups and the anti-CCP2– group). Anti-CCP2 anti-cyclic citrullinated peptide-2 antibodies, anti-CCP2– anti-CCP2 antibody negative, anti-CCP2+ anti-CCP2 antibody positive, DXR–BMD digital X-ray radiogrammetry–bone mineral density, Gp group
Associations Between DXR–BMD and Anti-CCP2 Antibody Status: Multivariate Analysis
When anti-CCP2 antibody level was used as a continuous variable, combined hand DXR–BMD was negatively associated with anti-CCP2. For every 10-unit increase in anti-CCP2 antibody level, DXR–BMD decreased by 0.0014 units (P < 0.001; see Supplementary Table 1). The overall model fit based on adjusted R2 for the total hand DXR–BMD model was 0.406. When anti-CCP2 antibody status was used as a categorical variable, combined hand DXR–BMD was associated with anti-CCP2+ Gp1–3 vs. anti-CCP2− (P < 0.001; Table 2). Combined hand DXR–BMD was negatively associated with each individual anti-CCP2 antibody titer group (Gp1, Gp2, or Gp3) vs. anti-CCP2− (P < 0.05). Adjusted R2 for the total hand DXR–BMD model was 0.426. This negative association between DXR–BMD and each individual anti-CCP2 antibody titer group vs. anti-CCP2− remained significant in the multivariate analysis (Table 2).
Results for individual hands were similar to those for the combined analysis (Table 2 and Supplementary Table 1). When anti-CCP2 antibody level was used as a continuous variable, for every 10-unit increase in anti-CCP2, DXR–BMD for the left or right hand decreased by 0.0014 units (P < 0.001; see Supplementary Table 1). Similarly, when anti-CCP2 antibody status was used as a categorical variable, left or right hand DXR–BMD was associated with anti-CCP2+ Gp1–Gp3 vs. anti-CCP2− (P < 0.001; Table 2).
DXR–BMD was negatively associated with age, duration of RA, and use of osteoporosis medication. In the model with anti-CCP2 antibody status as a categorical variable, steroid use > 6 months was also a significant factor for DXR–BMD (left or average; Table 2).
Association Between Disease Activity and Bone Loss
Evaluation of the association between DXR–BMD and disease activity indicates that patients with low DXR–BMD were less likely to have a DAS28 (CRP) < 2.6 (DXR–BMD ≥ 0.5, 36.5% vs. DXR–BMD < 0.5, 18.8%; P = 0.0181) (Fig. 3). After controlling for confounding factors, the odds of having a DAS28 (CRP) < 2.6 were significantly lower for patients with DXR–BMD < 0.5 (n = 64) vs. ≥ 0.5 (n = 85; odds ratio 0.355 [95% CI 0.126–0.998]; P = 0.0496).
Discussion
Our results show that, among patients with long-standing RA, hand DXR–BMD is negatively associated with the presence of anti-CCP2 antibodies. Patients with anti-CCP2+ status, particularly those with high anti-CCP2 antibody titers, had lower hand BMD; therefore, as anti-CCP2 antibody titers increased, hand BMD decreased. This is consistent with previous studies in patients with early RA, demonstrating a correlation between elevated anti-CCP2 antibody baseline levels and DXR–BMD loss [23, 24].
In the present analysis, patients with low DXR–BMD were less likely to have low disease activity. Similar observations have also been reported in patients with early RA [30], suggesting an association between disease activity and bone loss. Hand BMD loss has also been shown to indicate an increased risk of erosive disease [10, 11, 31, 32]. Data from an observational study demonstrated that BMD loss at 6 months was associated with higher erosion scores, and a higher proportion of patients with BMD loss at 6 months had at least one erosion and a higher risk of erosion progression at 12 months [31]. Furthermore, although there is evidence that hand joint damage in RA is related to use and hand dominance [33], our data show that bone loss occurs in both hands, which is consistent with RA being defined as a symmetrical disease. Such patients with low hand BMD may be at an increased risk of vertebral and non-vertebral fractures [6, 7].
Pro-inflammatory cytokines are generally the key drivers of articular and extra-articular bone damage [34,35,36]. However, recent evidence has shown that RA-associated autoantibodies, such as ACPA, can directly induce bone loss by stimulating osteoclast differentiation [20, 21]. In vivo, human ACPA causes bone loss in immune-deficient mice [20]. ACPA has been shown to be associated with bone loss as demonstrated through DXR–BMD in this study as well as DXA–BMD in a separate study [24]. Patients with ACPA develop cortical thinning, leading to a decrease in bone mass and increasing the risk of bone erosions [3, 22]. Given the evidence suggesting that ACPA is a key driver of bone loss [23, 24], treatment options for RA that reduce ACPA titers and induce seroconversion may be effective in lowering the risk of bone loss. This should be explored in future clinical trials.
The strength of this analysis is that these data are from an observational cohort of patients with RA, including clinical measures such as serological status and DXR–BMD. Limitations of this analysis include those inherent in observational cross-sectional studies, including the absence of a comparator (e.g., DXR–BMD in healthy, postmenopausal women) and hand radiographs and ACPA testing may not have been done on the same day. Even though our statistical models controlled for several covariates and observed significant relationships between DXR–BMD and ACPA or disease activity, this does not imply causation or rule out certain biases without further controlled analyses. Confounding by unmeasured variables should also be considered when evaluating these results. The selection of < 0.5 as compared with ≥ 0.5 g/cm2 in relation to DAS28 (CRP) < 2.6 should also be considered as a potential limitation. However, the cutoff was selected in reference to the DXR–BMD median of Gp3 and needs further validation in future studies. In addition, antibodies to individual citrullinated proteins (e.g. fibrin, filaggrin, vimentin) or other serological markers (e.g., rheumatoid factor) were not evaluated. The patient population primarily reflects postmenopausal women; therefore, future studies should be conducted in pre- and postmenopausal women as these results may have implications for osteoporosis prevention.
Conclusions
Our results show that, in routine clinical practice, anti-CCP2 antibody positivity in patients with established RA is associated with lower hand BMD, and patients with hand bone loss were less likely to have low disease activity. This suggests that DXR–BMD and anti-CCP2 antibody status could help identify patients at risk for joint progression and fracture; however, a direct causal relationship cannot necessarily be implied from this cross-sectional analysis. Disease-modifying treatment for RA that not only targets inflammation but improves cortical bone density should be considered in order to achieve better prevention of bone erosions in patients with RA.
References
Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumatol. 1988;31:315–24.
van der Heijde DM. Radiographic imaging: the ‘gold standard’ for assessment of disease progression in rheumatoid arthritis. Rheumatology (Oxford). 2000;39(Suppl 1):9–16.
Rossini M, Bagnato G, Frediani B, et al. Relationship of focal erosions, bone mineral density, and parathyroid hormone in rheumatoid arthritis. J Rheumatol. 2011;38:997–1002.
Hoff M, Kvien TK, Kalvesten J, et al. Adalimumab reduces hand bone loss in rheumatoid arthritis independent of clinical response: subanalysis of the PREMIER study. BMC Musculoskelet Disord. 2011;12:54.
Orsolini G, Caimmi C, Viapiana O, et al. Titer-dependent effect of anti-citrullinated protein antibodies on systemic bone mass in rheumatoid arthritis patients. Calcif Tissue Int. 2017;101:17–23.
Bouxsein ML, Palermo L, Yeung C, et al. Digital X-ray radiogrammetry predicts hip, wrist and vertebral fracture risk in elderly women: a prospective analysis from the study of osteoporotic fractures. Osteoporos Int. 2002;13:358–65.
Haugeberg G, Lodder MC, Lems WF, et al. Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50–70 year old female patients with rheumatoid arthritis: cross sectional Oslo–Truro–Amsterdam (OSTRA) collaborative study. Ann Rheum Dis. 2004;63:1331–4.
Book C, Algulin J, Nilsson JA, et al. Bone mineral density in the hand as a predictor for mortality in patients with rheumatoid arthritis. Rheumatology (Oxford). 2009;48:1088–91.
Jensen T, Hansen M, Jensen KE, et al. Comparison of dual X-ray absorptiometry (DXA), digital X-ray radiogrammetry (DXR), and conventional radiographs in the evaluation of osteoporosis and bone erosions in patients with rheumatoid arthritis. Scand J Rheumatol. 2005;34:27–33.
Kapetanovic MC, Lindqvist E, Algulin J, et al. Early changes in bone mineral density measured by digital X-ray radiogrammetry predict up to 20 years radiological outcome in rheumatoid arthritis. Arthritis Res Ther. 2011;13:R31.
Hoff M, Haugeberg G, Odegard S, et al. Cortical hand bone loss after 1 year in early rheumatoid arthritis predicts radiographic hand joint damage at 5-year and 10-year follow-up. Ann Rheum Dis. 2009;68:324–9.
Rezaei H, Saevarsdottir S, Geborek P, et al. Evaluation of hand bone loss by digital X-ray radiogrammetry as a complement to clinical and radiographic assessment in early rheumatoid arthritis: results from the SWEFOT trial. BMC Musculoskelet Disord. 2013;14:79.
Guler-Yuksel M, Allaart CF, Goekoop-Ruiterman YP, et al. Changes in hand and generalised bone mineral density in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2009;68:330–6.
Hoff M, Kvien TK, Kalvesten J, et al. Adalimumab therapy reduces hand bone loss in early rheumatoid arthritis: explorative analyses from the PREMIER study. Ann Rheum Dis. 2009;68:1171–6.
Krieckaert CL, Nurmohamed MT, Wolbink G, et al. Changes in bone mineral density during long-term treatment with adalimumab in patients with rheumatoid arthritis: a cohort study. Rheumatology (Oxford). 2013;52:547–53.
Vis M, Havaardsholm EA, Haugeberg G, et al. Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFkappaB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1495–9.
Eekman DA, Vis M, Bultink IE, et al. Stable bone mineral density in lumbar spine and hip in contrast to bone loss in the hands during long-term treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:389–90.
van der Helm-van Mil AH, Verpoort KN, Breedveld FC, et al. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R949–58.
Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81.
Harre U, Georgess D, Bang H, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122:1791–802.
Krishnamurthy A, Joshua V, Haj HA, et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis. 2016;75:721–9.
Kleyer A, Finzel S, Rech J, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2014;73:854–60.
Boyesen P, Hoff M, Odegard S, et al. Antibodies to cyclic citrullinated protein and erythrocyte sedimentation rate predict hand bone loss in patients with rheumatoid arthritis of short duration: a longitudinal study. Arthritis Res Ther. 2009;11:R103.
Bugatti S, Bogliolo L, Vitolo B, et al. Anti-citrullinated protein antibodies and high levels of rheumatoid factor are associated with systemic bone loss in patients with early untreated rheumatoid arthritis. Arthritis Res Ther. 2016;18:226.
Bykerk VP, Shadick N, Frits M, et al. Flares in rheumatoid arthritis: frequency and management. A report from the BRASS registry. J Rheumatol. 2014;41:227–34.
Iannaccone CK, Lee YC, Cui J, et al. Using genetic and clinical data to understand response to disease-modifying anti-rheumatic drug therapy: data from the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study. Rheumatology (Oxford). 2011;50:40–6.
Iannaccone CK, Fossel A, Tsao H, et al. Factors associated with attrition in a longitudinal rheumatoid arthritis registry. Arthritis Care Res (Hoboken). 2013;65:1183–9.
Desai SP, Gravallese EM, Shadick NA, et al. Hand bone mineral density is associated with both total hip and lumbar spine bone mineral density in post-menopausal women with RA. Rheumatology (Oxford). 2010;49:513–9.
Hochberg Y. A sharper Bonferroni procedure for multiple significance testing. Biometrika. 1988;75:800–3.
Forslind K, Kalvesten J, Hafstrom I, et al. Does digital X-ray radiogrammetry have a role in identifying patients at increased risk for joint destruction in early rheumatoid arthritis? Arthritis Res Ther. 2012;14:R219.
Black RJ, Spargo L, Schultz C, et al. Decline in hand bone mineral density indicates increased risk of erosive change in early rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66:515–22.
Bejarano V, Hensor E, Green M, et al. Relationship between early bone mineral density changes and long-term function and radiographic progression in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:66–70.
Koh JH, Jung SM, Lee JJ, et al. Radiographic structural damage is worse in the dominant than the non-dominant hand in individuals with early rheumatoid arthritis. PLoS One. 2015;10:e0135409.
Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev. 2010;233:301–12.
Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656–64.
Bugatti S, Manzo A, Caporali R, et al. Assessment of synovitis to predict bone erosions in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2012;4:235–44.
Acknowledgements
Funding
This study was funded by Bristol-Myers Squibb (funding for the completion of the analysis, the development of the manuscript, and all publication charges and open access fees). All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Medical Writing, Editorial, and Other Assistance
Professional medical writing and editorial assistance was provided by Stacey Reeber, PhD, at Caudex and was funded by Bristol-Myers Squibb.
Disclosures
Harris A. Ahmad is an employee of and has stock options/bond holdings in Bristol-Myers Squibb. Evo Alemao is an employee of and has stock options/bond holdings in Bristol-Myers Squibb. Zhenchao Guo is an employee of and has stock options/bond holdings in Bristol-Myers Squibb. Michael Weinblatt has received grant/research support from Bristol-Myers Squibb, Crescendo Bioscience, UCB, and Amgen; and is a consultant (all less than $10,000) for Amgen, Bristol-Myers Squibb, Crescendo Bioscience, and UCB. Nancy A. Shadick has received grant/research support from Amgen, Bristol-Myers Squibb, Crescendo Bioscience, Mallinckrodt, UCB; and is a consultant (less than $10,000) for Bristol-Myers Squibb. Christine K. Iannaccone and Michelle L. Frits have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol and informed consent document were reviewed and approved by the Brigham and Women’s Hospital Institutional Review Board (approval number 2002P001763). All patients provided written informed consent before participating in the BRASS Registry.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/2F1DF0604CB189A2.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ahmad, H.A., Alemao, E., Guo, Z. et al. Association of Low Bone Mineral Density with Anti-Citrullinated Protein Antibody Positivity and Disease Activity in Established Rheumatoid Arthritis: Findings from a US Observational Cohort. Adv Ther 35, 232–242 (2018). https://doi.org/10.1007/s12325-017-0657-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-017-0657-x