Abstract
Objective
To review the teratogenic effects associated with the use of Food and Drug Administration-approved agents for bipolar disorder.
Methods
A PubMed search of all English language articles published from January 1966 to December 2008 was conducted. The key search terms included all major bipolar agents, cross-referenced with: teratogenicity, teratogen, safety, pregnancy, fetus, bipolar disorder, and malformation. The search was augmented with manual reviews of relevant article reference lists as well as http://clinicaltrials.gov and http://www.fda.gov (both last accessed in April 2008). Several pregnancy registries were also reviewed to determine malformation rates as well as teratogenesis attributable to each agent. Articles selected for review were based on author consensus, adequacy of sample size, the use of standardized experimental procedures, validated assessment measures, and overall manuscript quality.
Results
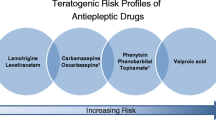
Valproate is associated with the highest rate of major congenital malformations (6.2%–16%). The relative risk of neural tube defects with valproate and carbamazepine is reported as approximately 1%–5% and 0.5%–1%, respectively. Preliminary evidence suggests that the relative risk for oral clefts (cleft lip or palate) is increased with lamotrigine relative to other antiepileptic drugs (AED) (ie, approximately 0.4%). The rate of major congenital malformations is higher in fetuses exposed to AED polytherapy (ie, ≥2 drugs) in comparison with AED monotherapy. Adverse neurobehavioral effects are insufficiently reported for most agents. In-utero exposure to valproate is associated with a greater risk of developmental difficulty requiring special education interventions as well as decreased verbal IQ scores. The risk of Ebstein’s anomaly associated with lithium use is increased relative to the general population. The major congenital malformation rate with chlorpromazine and atypical antipsychotics is not established as being higher than a non-exposed group; the teratogenic risks associated with the olanzapine-fluoxetine combination are unknown.
Conclusions
Well-characterized risks are associated with valproate, carbamazepine, lamotrigine, and lithium. The risks associated with psychotropic drug use need to be understood in the context of significant rates of relapse and associated morbidity when discontinuing bipolar treatment during pregnancy.
Similar content being viewed by others
References
Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCSR). JAMA. 2003;289:3095–3105.
Tondo L, Isacsson G, Baldessarini R. Suicidal behaviour in bipolar disorder: risk and prevention. CNS Drugs. 2003;17:491–511.
Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition Text revision (DSM-IV-TR). 4th edition. Washington: American Psychiatric Association; 2000.
McIntyre RS, Soczynska JK, Konarski JZ, et al. Should depressive syndromes be reclassified as “Metabolic Syndrome Type II”? Ann Clin Psychiatry. 2007;19:257–264.
McIntyre RS, Konarski JZ. Bipolar disorder: a national health concern. CNS Spectr. 2004;9:6–15.
McIntyre R, Kennedy S, Bagby RM, Bakish D. Assessing full remission. J Psychiatry Neurosci. 2002;27:235–239.
Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164:1817–1824.
Pandarakalam JP. Clinical challenges of bipolar depression. Br J Hosp Med (Lond). 2007;68:530–537.
Ernst CL, Goldberg JF. The reproductive safety profile of mood stabilizers, atypical antipsychotics, and broad-spectrum psychotropics. J Clin Psychiatry. 2002;63(suppl. 4):42–55.
Yonkers KA, Wisner KL, Stowe Z, et al. Management of bipolar disorder during pregnancy and the postpartum period. Am J Psychiatry. 2004;161:608–620.
Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord. 2006;8:721–739.
Viguera AC, Koukopoulos A, Muzina DJ, et al. Teratogenicity and anticonvulsants: lessons from neurology to psychiatry. J Clin Psychiatry. 2007;68(suppl. 9):29–33.
Koren G, Cohn T, Chitayat D, et al. Use of atypical antipsychotics during pregnancy and the risk of neural tube defects in infants. Am J Psychiatry. 2002;159:136–137.
Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407–412.
Wide K, Winbladh B, Kallen B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register study. Acta Paediatr. 2004;93:174–176.
Burt VK, Rasgon N. Special considerations in treating bipolar disorder in women. Bipolar Disord. 2004;6:2–13.
Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–198.
Kaneko S, Battino D, Andermann E, et al. Congenital malformations due to antiepileptic drugs. Epilepsy Res. 1999;33:145–158.
Kaaja E, Kaaja R, Hiilesmaa V. Major malformations in offspring of women with epilepsy. Neurology. 2003;60:575–579.
Artama M, Auvinen A, Raudaskoski T, et al. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64:1874–1878.
Vajda FJ, O’Brien TJ, Hitchcock, A et al. The Australian registry of anti-epileptic drugs in pregnancy: experience after 30 months. J Clin Neurosci. 2003;10:543–549.
Wyszynski DF, Nambisan M, Surve T, et al. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64:961–965.
Vajda FJ, Eadie MJ. Maternal valproate dosage and foetal malformations. Acta Neurol Scand. 2005;112:137–143.
Vajda FJ, O’Brien TJ, Hitchcock A, et al. Critical relationship between sodium valproate dose and human teratogenicity: results of the Australian register of anti-epileptic drugs in pregnancy. J Clin Neurosci. 2004;11:854–858.
Vajda FJ, Hitchcock A, Graham J, et al. Foetal malformations and seizure control: 52 months data of the Australian Pregnancy Registry. Eur J Neurol. 2006;13:645–654.
Cunnington M, Tennis P. Lamotrigine and the risk of malformations in pregnancy. Neurology. 2005;64:955–960.
Holmes LB, Harvey EA, Coull BA, et al. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344:1132–1138.
Samren EB, van Duijn CM, Christiaens GC, et al. Antiepileptic drug regimens and major congenital abnormalities in the offspring. Ann Neurol. 1999;46:739–746.
Canger R, Battino D, Canevini MP, et al. Malformations in offspring of women with epilepsy: a prospective study. Epilepsia. 1999;40:1231–1236.
Adab N, Jacoby A, Smith D, et al. Additional educational needs in children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2001;70:15–21.
Cohen LS, Friedman JM, Jefferson JW, et al. A reevaluation of risk of in utero exposure to lithium. JAMA. 1994;271:146–150.
Schou M, Goldfield MD, Weinstein MR, et al. Lithium and pregnancy. I. Report from the Register of Lithium Babies. Br Med J. 1973;2:135–136.
McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66:444–449.
Goldstein DJ, Corbin LA, Fung MC. Olanzapineexposed pregnancies and lactation: early experience. J Clin Psychopharmacol. 2000;20:399–403.
Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1575–1583.
Vinten J, Adab N, Kini U, et al. Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology. 2005;64:949–954.
Gaily E, Kantola-Sorsa E, Hiilesmaa V, et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology. 2004;62:28–32.
Olafsson E, Hallgrimsson JT, Hauser WA, et al. Pregnancies of women with epilepsy: a population-based study in Iceland. Epilepsia. 1998;39:887–892.
Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Seizure. 2008;17:166–171.
Llewellyn A, Stowe ZN, Strader JR Jr. The use of lithium and management of women with bipolar disorder during pregnancy and lactation. J Clin Psychiatry. 1998;59(suppl. 6):57–64.
Nonacs R, Cohen LS. Assessment and treatment of depression during pregnancy: an update. Psychiatr Clin North Am. 2003;26:547–562.
Jones KL, Lacro RV, Johnson KA, et al. Pattern of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med. 1989;320:1661–1666.
Weinstein MR. The international register of lithium babies. Drug Inf J. 1976;10:94–100.
DiLiberti JH, Farndon PA, Dennis NR, et al. The fetal valproate syndrome. Am J Med Genet. 1984;19:473–481.
Mackay FJ, Wilton LV, Pearce GL, et al. Safety of long-term lamotrigine in epilepsy. Epilepsia. 1997;38:881–886.
CPS. Compendium of Pharmaceuticals and Specialties. Ottawa: Canadian Pharmacist Association; 2007.
Auerbach JG, Hans SL, Marcus J, et al. Maternal psychotropic medication and neonatal behavior. Neurotoxicol Teratol. 1992;14:399–406.
Ratnayake T, Libretto SE. No complications with risperidone treatment before and throughout pregnancy and during the nursing period. J Clin Psychiatry. 2002;63:76–77.
Titze K, Koch S, Helge H, et al. Prenatal and family risks of children born to mothers with epilepsy: effects on cognitive development. Dev Med Child Neurol. 2008;50:117–122.
Moore SJ, Turnpenny P, Quinn A, et al. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet. 2000;37:489–497.
Rasalam AD, Hailey H, Williams JH, et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol. 2005;47:551–555.
Williams PG, Hersh JH. A male with fetal valproate syndrome and autism. Dev Med Child Neurol. 1997;39:632–634.
Yerby MS. Management issues for women with epilepsy: neural tube defects and folic acid supplementation. Neurology. 2003;61(6 suppl. 2):S23–S26.
Viguera AC, Cohen LS. The course and management of bipolar disorder during pregnancy. Psychopharmacol Bull. 1998;34:339–346.
Kallen AJ. Maternal carbamazepine and infant spina bifida. Reprod Toxicol. 1994;8:203–205.
Matalon S, Schechtman S, Goldzweig G, et al. The teratogenic effect of carbamazepine: a meta-analysis of 1255 exposures. Reprod Toxicol. 2002;16:9–17.
Nulman I, Scolnik D, Chitayat D, et al. Findings in children exposed in utero to phenytoin and carbamazepine monotherapy: independent effects of epilepsy and medications. Am J Med Genet. 1997;68:18–24.
Rosa FW. Spina bifida in infants of women treated with carbamazepine during pregnancy. N Engl J Med. 1991;324:674–677.
av-Citrin O, Shechtman S, Arnon J, et al. Is carbamazepine teratogenic? A prospective controlled study of 210 pregnancies. Neurology. 2001;57:321–324.
Iqbal MM, Gundlapalli SP, Ryan WG, et al. Effects of antimanic mood-stabilizing drugs on fetuses, neonates, and nursing infants. South Med J. 2001;94:304–322.
Holmes LB, Coull BA, Dorfman J, et al. The correlation of deficits in IQ with midface and digit hypoplasia in children exposed in utero to anticonvulsant drugs. J Pediatr. 2005;146:118–122.
Gentile S. Prophylactic treatment of bipolar disorder in pregnancy and breastfeeding: focus on emerging mood stabilizers. Bipolar Disord. 2006;8:207–220.
Prakash, Prabhu LV, Nasar MA, et al. Lamotrigine in pregnancy: safety profile and the risk of malformations. Singapore Med J. 2007;48:880–883.
Shor S, Koren G, Nulman I. Teratogenicity of lamotrigine. Can Fam Physician. 2007;53:1007–1009.
Newport DJ, Stowe ZN, Viguera AC, et al. Lamotrigine in bipolar disorder: efficacy during pregnancy. Bipolar Disord. 2008;10:432–436.
Newport DJ, Viguera AC, Beach AJ, et al. Lithium placental passage and obstetrical outcome: implications for clinical management during late pregnancy. Am J Psychiatry. 2005;162:2162–2170.
Newport DJ, Calamaras MR, DeVane CL, et al. Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. Am J Psychiatry. 2007;164:1214–1220.
Dube S, Tollefson GD, Thase ME, et al. Onset of antidepressant effect of olanzapine and olanzapine/fluoxetine combination in bipolar depression. Bipolar Disord. 2007;9:618–627.
Alwan S, Reefhuis J, Rasmussen SA, et al. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356:2684–2692.
Louik C, Lin AE, Werler MM, et al. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356:2675–2683.
Eadie MJ. Antiepileptic drugs as human teratogens. Expert Opin Drug Saf. 2008;7:195–209.
Fried S, Kozer E, Nulman I, et al. Malformation rates in children of women with untreated epilepsy: a meta-analysis. Drug Saf. 2004;27:197–202.
Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157:179–184.
Samren EB, van Duijn CM, Koch S, et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38:981–990.
McIntyre RS, McCann SM, Kennedy SH. Antipsychotic metabolic effects: weight gain, diabetes mellitus, and lipid abnormalities. Can J Psychiatry. 2001;46:273–281.
McIntyre RS. Psychotropic drugs and adverse events in the treatment of bipolar disorders revisited. J Clin Psychiatry. 2002;63(suppl. 3):15–20.
Ward S, Wisner KL. Collaborative management of women with bipolar disorder during pregnancy and postpartum: pharmacologic considerations. J Midwifery Womens Health. 2007;52:3–13.
Carpenter MW. Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care. 2007;30(suppl. 2):S246–S250.
Carmichael SL, Shaw GM, Neri E, et al. Physical activity and risk of neural tube defects. Matern Child Health J. 2002;6:151–157.
Rambeck B, Kurlemann G, Stodieck SR, et al. Concentrations of lamotrigine in a mother on lamotrigine treatment and her newborn child. Eur J Clin Pharmacol. 1997;51:481–484.
Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994;45:444–450.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, H.T.T., Sharma, V. & McIntyre, R.S. Teratogenesis associated with antibipolar agents. Adv Therapy 26, 281–294 (2009). https://doi.org/10.1007/s12325-009-0011-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-009-0011-z