Abstract
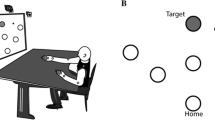
Damage to the cerebellum causes a disabling movement disorder called ataxia, which is characterized by poorly coordinated movement. Arm ataxia causes dysmetria (over- or under-shooting of targets) with many corrective movements. As a result, people with cerebellar damage exhibit reaching movements with highly irregular and prolonged movement paths. Cerebellar patients are also impaired in error-based motor learning, which may impede rehabilitation interventions. However, we have recently shown that cerebellar patients can learn a simple reaching task using a binary reinforcement paradigm, in which feedback is based on participants’ mean performance. Here, we present a pilot study that examined whether patients with cerebellar damage can use this reinforcement training to learn a more complex motor task—to decrease the path length of their reaches. We compared binary reinforcement training to a control condition of massed practice without reinforcement feedback. In both conditions, participants made target-directed reaches in 3-dimensional space while vision of their movement was occluded. In the reinforcement training condition, reaches with a path length below participants’ mean were reinforced with an auditory stimulus at reach endpoint. We found that patients were able to use reinforcement signaling to significantly reduce their reach paths. Massed practice produced no systematic change in patients’ reach performance. Overall, our results suggest that binary reinforcement training can improve reaching movements in patients with cerebellar damage and the benefit cannot be attributed solely to repetition or reduced visual control.





Similar content being viewed by others
References
Holmes G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain. 1917;40:461–535.
Bastian A, Thach W. Cerebellar outflow lesions: a comparison of movement deficits resulting from lesions at the levels of the cerebellum and thalamus. Ann Neurol. 1995;38:881–92.
Bastian A, Martin T, Keating J, Thach W. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509.
Bhanpuri N, Okamura A, Bastian A. Predicting and correcting ataxia using a model of cerebellar function. Brain. 2014;137:1931–44.
Therrien A, Bastian A. The cerebellum as a movement sensor. Neurosci Lett. 2018;688:37–40.
Krakauer J, Pine Z, Ghilardi M, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci. 2000;20:8916–24.
Shadmehr R, Smith M, Krakauer J. Error correction, sensory prediction, and adaptation in motor control. Neuroscience. 2010;33:89–108.
Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology. 1983;33:766–72.
Martin T, Keating J, Goodkin H, Bastian A, Thach W. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119:1183–98.
Maschke M, Gomez C, Ebner T, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol. 2004;91:230–8.
Smith M, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–21.
Tseng Y, Diedrichsen J, Krakauer J, Shadmehr R, Bastian A. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62.
Schlerf J, Xu J, Klemfuss N, Griffiths T, Ivry R. Individuals with cerebellar degeneration show similar adaptation deficits with large and small visuomotor errors. J Neurophysiol. 2013;109:1164–73.
Therrien A, Wolpert D, Bastian A. Effective reinforcement learning following cerebellar damage requires a balance between exploration and motor noise. Brain. 2016. 2016;139:101–14.
Izawa J, Criscimagna-Hemminger S, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci. 2012;32:4230–9.
Sutton RS, Barto G. An introduction to reinforcement learning. Cambridge: MIT Press; 1998.
Lee D, Seo H, Jung M. Neural basis of reinforcement learning and decision making. Ann Rev Neurosci. 2012;35:287–308.
Izawa J, Shadmehr R. Learning from sensory and reward prediction errors during motor adaptation. PLoS Comp Biol. 2011;7:e1002012.
Trouillas P, Takayanagi T, Hallett M, Currier R, Subramony S, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. J Neurol Sci. 1997;145:205–11.
Bastian A. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16:645–9.
Zimmet A, Bastian A, Cowan N. Cerebellar patients have intact feedback control that can be leveraged to improve reaching. bioRxiv. 2019.
Ilg W, Bastian A, Boesch S, Burciu R, Celnik P, Claasen J, et al. Consensus paper: management of degenerative cerebellar disorders. Cerebellum. 2014;13:248–68.
Uehara S, Mawase F, Therrien A, Cherry-Allen K, Celnik P. Interactions between motor exploration and reinforcement learning. J Neurophysiol. 2019;122:797–808.
Cashaback J, McGregor H, Mohatarem A, Gribble P. Dissociating error-based and reinforcement-based loss functions during sensorimotor learning. PLOS Comp Biol. 2017;13:e1005623.
Wong A, Marvel C, Taylor J, Krakauer J. Can patients with cerebellar disease switch learning mechanisms to reduce their adaptation deficits? bioRxiv. 2018.
Beppu H, Suda M, Tanaka R. Analysis of cerebellar motor disorders by visually guided elbow tracking movement. Brain. 1984;107:787–809.
Day B, Thompson P, Harding A, Marsden C. Influence of vision on upper limb reaching movements in patients with cerebellar ataxia. Brain. 1998;121:357–72.
Miall R, Christensen L, Cain O, Stanley J. Disruption of state estimation in the human lateral cerebellum. PLoS Biol. 2007;5:e316.
Mazzoni P, Krakauer J. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci. 2006;26:3642–5.
Benson B, Anguera J, Seidler R. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J Neurophysiol. 2011;105:2843–51.
Taylor J, Ivry R. Flexible cognitive strategies during motor learning. PLoS Comp Biol. 2011;7:e1001096.
McDougle S, Bond K, Taylor J. Explicit and implicit processes constitute the fast and slow processes of sensorimotor learning. J Neurosci. 2015;35:9568–79.
Schween R, McDougle S, Hegele M, Taylor J. Assessing explicit strategies in force field adaptation. J Neurophysiol. 2020;123:1552–65.
Codol O, Holland P, Galea J. The relationship between reinforcement and explicit control during visuomotor adaptation. Sci Rep. 2018;8:9121.
Holland P, Codol O, Galea J. Contribution of explicit processes to reinforcement-based motor learning. J Neurophysiol. 2018;119:2241–55.
Butcher P, Ivry R, Kuo S, Rydz D, Krakauer J, Taylor J. The cerebellum does more than sensory prediction error-based learning in sensorimotor adaptation tasks. J Neurophysiol. 2017;118:1622–36.
McDougle S, Tsay J, Taylor J, Ivry R. Cerebellar degeneration selectively disrupts continuous mental operations in visual cognition. bioRxiv. 2020.
Campbell WW. DeJong’s the neurologic examination. Baltimore: Lippincott Williams and Wilkins; 2005.
Funding
This work was supported by the National Institute of Child Health and Human Development HD040289 to AJB and a Johns Hopkins Distinguished Science of Learning Fellowship to AST.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Therrien, A.S., Statton, M.A. & Bastian, A.J. Reinforcement Signaling Can Be Used to Reduce Elements of Cerebellar Reaching Ataxia. Cerebellum 20, 62–73 (2021). https://doi.org/10.1007/s12311-020-01183-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-020-01183-x