Abstract
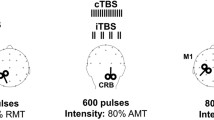
The cerebellum is implicated in the pathophysiology of numerous movement disorders, which makes it an attractive target for noninvasive neurostimulation. Continuous theta burst stimulation (cTBS) can induce long lasting plastic changes in human brain; however, the efficacy of different simulation protocols has not been investigated at the cerebellum. Here, we compare a traditional 50-Hz and a modified 30-Hz cTBS protocols at modulating cerebellar activity in healthy subjects. Seventeen healthy adults participated in two testing sessions where they received either 50-Hz (cTBS50) or 30-Hz (cTBS30) cerebellar cTBS. Cerebellar brain inhibition (CBI), a measure of cerebello-thalamocortical pathway strength, and motor evoked potentials (MEP) were measured in the dominant first dorsal interosseous muscle before and after (up to ~ 40 min) cerebellar cTBS. Both cTBS protocols induced cerebellar depression, indicated by significant reductions in CBI (P < 0.001). No differences were found between protocols (cTBS50 and cTBS30) at any time point (P = 0.983). MEP amplitudes were not significantly different following either cTBS protocol (P = 0.130). The findings show cerebellar excitability to be equally depressed by 50-Hz and 30-Hz cTBS in heathy adults and support future work to explore the efficacy of different cerebellar cTBS protocols in movement disorder patients where cerebellar depression could provide therapeutic benefits.




Similar content being viewed by others
References
Chung SW, Hill AT, Rogasch NC, Hoy KE, Fitzgerald PB. Use of theta-burst stimulation in changing excitability of motor cortex: a systematic review and meta-analysis. Elsevier Ltd 2016;1–22.
Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6.
Suppa A, Huang YZ, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, et al. Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Elsevier Inc 2016;1–13.
Wischnewski M, Schutter DJLG. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimulation. Elsevier Inc 2015; 8:685–92.
Nyffeler T, Wurtz P, Lüscher H-R, Hess CW, Senn W, Pflugshaupt T, et al. Repetitive TMS over the human oculomotor cortex: comparison of 1-Hz and theta burst stimulation. Neurosci Lett. 2006;409:57–60.
Nyffeler T, Cazzoli D, Wurtz P, Lüthi M, von Wartburg R, Chaves S, et al. Neglect-like visual exploration behaviour after theta burst transcranial magnetic stimulation of the right posterior parietal cortex. Eur J Neurosci. 2008;27:1809–13.
Wu SW, Shahana N, Huddleston DA, Gilbert DL. Effects of 30Hz theta burst transcranial magnetic stimulation on the primary motor cortex. Journal of Neuroscience Methods Elsevier B.V 2012; 208:161–4.
Goldsworthy MR, Pitcher JB, Ridding MC. A comparison of two different continuous theta burst stimulation paradigms applied to the human primary motor cortex. Clin Neurophysiol. 2012;123:2256–63.
Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimulation Elsevier Inc. 2010;3:161–9.
Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SNF, Gerwig M, et al. Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2nd ed. 2011; 11:457–87.
Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain. 2013;136:696–709.
Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends in Cognitive Sciences Elsevier Ltd. 2013;17:241–54.
Koch G, Mori F, Marconi B, Codecà C, Pecchioli C, Salerno S, et al. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol. 2008;119:2559–69.
Celnik P. Understanding and modulating motor learning with cerebellar stimulation. Cerebellum. 2014;14:171–4.
Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann. Neurol. Wiley subscription services, Inc.. A Wiley Company; 1995; 37:703–13.
Lam CK, Staines WR, Tokuno CD, Bent LR. The medium latency muscle response to a vestibular perturbation is increased after depression of the cerebellar vermis. Brain Behav. 2017;7:e00782–9.
Koch G, Brusa L, Carrillo F, Gerfo Lo E, Torriero S, Oliveri M, et al. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009;73:113–9.
Koch G, Porcacchia P, Ponzo V, Carrillo F, Cáceres-Redondo MT, Brusa L, et al. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimulation Elsevier Ltd. 2014;7:564–72.
Hardwick RM, Lesage E, Miall RC. Cerebellar transcranial magnetic stimulation: the role of coil geometry and tissue depth. Brain Stimulation Elsevier Ltd. 2014;7:643–9.
Fernandez L, Major BP, Teo W-P, Byrne LK, Enticott PG. Assessing cerebellar brain inhibition (CBI) via transcranial magnetic stimulation (TMS): a systematic review. Neurosci Biobehav Rev. 2018;86:176–206.
Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75:1765–78.
Huang Y-Z, Rothwell JC, Chen R-S, Lu C-S, Chuang W-L. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2011;122:1011–8.
Huang Y-Z, Chen R-S, Rothwell JC, Wen H-Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–32.
Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21:827–35.
Molnar GF, Sailer A, Gunraj CA, Lang AE, Lozano AM, Chen R. Thalamic deep brain stimulation activates the cerebellothalamocortical pathway. Neurology. 2004;63:907–9.
Ni Z, Pinto AD, Lang AE, Chen R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol. 2010;68:816–24.
Carrillo F, Palomar FJ, Conde V, Diaz-Corrales FJ, Porcacchia P, Fernández-del-Olmo M, et al. Study of cerebello-thalamocortical pathway by transcranial magnetic stimulation in Parkinson’s disease. Brain Stimulation Elsevier Ltd. 2013;6:582–9.
Brighina F, Romano M, Giglia G, Saia V, Puma A, Giglia F, et al. Effects of cerebellar TMS on motor cortex of patients with focal dystonia: a preliminary report. Exp Brain Res. 2008;192:651–6.
Fernandez L, Major BP, Teo W-P, Byrne LK, Enticott PG. The impact of stimulation intensity and coil type on reliability and tolerability of cerebellar brain inhibition (CBI) via dual-coil TMS. Cerebellum. 2018;78:272–10.
Oliveri M, Koch G, Torriero S, Caltagirone C. Increased facilitation of the primary motor cortex following 1Hz repetitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci Lett. 2005;376:188–93.
Li Voti P, Conte A, Rocchi L, Bologna M, Khan N, Leodori G, et al. Cerebellar continuous theta-burst stimulation affects motor learning of voluntary arm movements in humans. Eur J Neurosci. 2013;39:124–31.
Bologna M, Di Biasio F, Conte A, Iezzi E, Modugno N, Berardelli A. Effects of cerebellar continuous theta burst stimulation on resting tremor in Parkinson’s disease. Parkinsonism and Related Disorders Elsevier Ltd. 2015;21:1061–6.
Helmich RC, Janssen MJR, Oyen WJG, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol. 2011;69:269–81.
Lefaivre SC, Brown MJN, Almeida QJ. Cerebellar involvement in Parkinson’s disease resting tremor. Cerebellum Ataxias. 2016;3:13.
Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol Lond. 2010;588:2291–304.
Pell GS, Roth Y, Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol Elsevier Ltd. 2011;93:59–98.
Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 2013;23:1593–605.
Acknowledgments
The authors would like to thank Dr. Oury Monchi for use of his laboratory space, and Rachel Sondergaard for assistance with data collection.
Funding
This work was supported in part by the Natural Sciences and Engineering Research Council of Canada Grant No. 2017-04126 (ZHTK), Eyes High University of Calgary fellowship, Parkinson Alberta fellowship, and Parkinson’s Foundation fellowship Grant No. PF-FBS-1776 (NDJS), Branch Out Neurological Foundation (ADC), as well as the Hotchkiss Brain Institute N3 Network.
Author information
Authors and Affiliations
Contributions
N.D.J.S, L. G, and Z.H.T.K conceived and designed the research; N.D.J.S, A.D.C, and L. G performed the experiments; N.D.J.S and A.D.C analyzed data and prepared figures; N.D.J.S drafted manuscript; N.D.J.S, A.D.C, L. G, and Z.H.T.K edited and revised manuscript; N.D.J.S, A.D.C, L. G, and Z.H.T.K approved final version of manuscript.
Corresponding author
Ethics declarations
The experimental procedures were approved by the University of Calgary Research Ethics Board and complied with the Declaration of Helsinki.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 1112 kb)
Rights and permissions
About this article
Cite this article
Strzalkowski, N.D.J., Chau, A.D., Gan, L.S. et al. Both 50 and 30 Hz continuous theta burst transcranial magnetic stimulation depresses the cerebellum. Cerebellum 18, 157–165 (2019). https://doi.org/10.1007/s12311-018-0971-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-018-0971-0